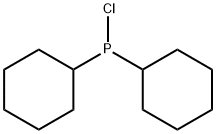
Synthesis and Applications of Dicyclohexylchlorophosphine
May 30,2025
Dicyclohexylchlorophosphine is used as pharmaceutical intermediate. It is also used in the synthesis of various phosphines. Latexin deletion protects against radiation-induced hematopoietic damages via selective activation of Bcl-2 prosurvival pathway.

Synthesis of Dicyclohexylchlorophosphine
A 1L three necked Schlenk flask equipped with a reflux condenser, and dropping funnel was loaded with Mg (25.59 g, 1.01 mol) and two or three small iodine crystals. The Schlenk flask was heated gently usinga heat gun, until all iodine had sublimed. Subsequently, 300 mL of diethyl ether were added at RT and a solution of chlorocyclohexane (80.00g, 80.00 mL, 0.675 mol) diethyl ether (120 mL) was added drop wise over the course of 2h. The suspension was subsequently refluxed for 1.5h under continuous stirring. A 2L Schlenk flask was then loaded with PCl3 (46.31g, 29.50 mL, 0.337 mol) and diethyl ether(800 mL), and cooled to -40°C. The freshly prepared Grignard solution was then filtered directly into thePCl3 solution, maintaining dropwise addition, strictly at -40°C over the course of 3h under continuous stirring (N.B. during the dropwise addition of the Grignard reagent large amounts of MgCl2 are formed).After all the Grignard reagent was added, the reaction mixture was allowed to warm to RT overnight. The reaction mixture was then filtered and all volatiles removed in vacuo, yielding a yellow liquid. The crude product was purified by fractional vacuum distillation (10-2 mbar), with the monosubstituted side product (i.e. Dicyclohexylchlorophosphine) evaporating at an oil bath temperature of 85-90°C and a vapor temperature of 50-55°C, and the product evaporating at an oil bath temperature of 105-110°C and a vapor temperature of80-85°C. Analytically pure Dicyclohexylchlorophosphine was isolated as a colourless liquid (65.74 g, 0.282 mmol, 83 %).[1]
Dicyclohexylchlorophosphine applied in Cr-catalysis
9-Amino-9-phosphabicyclo[3.3.1]nonanes, (PhobPNHR′; R = Me or iPr) are readily prepared by aminolysis of PhobPCl and are significantly less susceptible to hydrolysis than the acyclic analogues Cy2PNHR′. Treatment of Cy2PNHMe with Dicyclohexylchlorophosphine readily gave Cy2PNMePCy2. The unsymmetrical diphos ligands PhobPNMePAr2 (Ar = Ph, o-Tol) are prepared, converted to [Cr(CO)4(PhobPNMePAr2)] and shown to form Cr-catalysts for ethene oligomerisation, producing a pattern of higher alkenes that corresponds to a Schulz-Flory distribution overlaid on selective tri/tetramerisation. The previously reported diphosphinoamine L3 is readily prepared from MeNH2 and Dicyclohexylchlorophosphine in the presence of Et3N.[2]
The intermediate in this reaction is presumably Cy2PNHMe (L1) and indeed treatment of the isolated L1 with Dicyclohexylchlorophosphine in the presence of Et3N in CH2Cl2 gave L3 quantitatively according to 31P NMR spectroscopy. The spectrum of the reaction mixture also revealed a transient PPN species (as evidenced by a large JPP of 280 Hz) to which the tautomeric structure L′3 is assigned. In contrast to the ready reaction of L1 with Cy2PCl to give PNP ligand L3, the reaction of PhobPNHMe (La) with PhobPCl in the presence of NEt3 or N-methylpyrrolidine did not give the expected diphosphinoamine Lc. The reaction between PhobPNHMe and Dicyclohexylchlorophosphine was followed by 31P NMR spectroscopy and it was unambiguously shown that a PPN product was formed which, on the basis of its JPP of 358 Hz, was tentatively assigned to the protonated species L′e·HCl. The monodentate aminophobanes PhobPNHR (R = Me or iPr) have been readily prepared and are more resistant to hydrolysis than their Cy2PNHR analogues consistent with the PhobP group having a greater effective steric bulk than Cy2P. Attempts to make the free ligand PhobPNMePPhob have been thwarted by formation of PPN species which resist tautomerisation although a rearrangement takes place in the presence of [PdCl2(cod)] to give the desired PNP–Pd chelate. The readily prepared mixed diphos ligands PhobPNMePAr2 (Ar = Ph or o-Tol) in combination with Cr, catalysed the oligomerisation of ethylene with a partial selectivity to tri/tetramerisation, the remainder of the selectivity appearing to be Schulz-Flory in nature; the activities were moderate, but the polymer formation was high.
References
[1]Kalkuhl, Till L.; Fernández, Israel; Hadlington, Terrance J.[Chem, 2025, vol. 11, # 4, art. no. 102349]
[2]Haddow, Mairi F et al. “Aminophobanes: hydrolytic stability, tautomerism and application in Cr-catalysed ethene oligomerisation.” Dalton transactions (Cambridge, England : 2003) vol. 45,5 (2016): 2294-307. doi:10.1039/c5dt04394h
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringDibenzoylmethane is a metal-chelating agent with anti-proliferative and anticarcinogenic activities against various cancers.....
Jun 3,2025Chemical MaterialsDicyclohexylchlorophosphine
16523-54-9You may like
Dicyclohexylchlorophosphine manufacturers
- Dicyclohexylchlorophosphine
-
- $59.00 / 25g
- 2025-12-15
- CAS:16523-54-9
- Min. Order: 25g
- Purity: 0.98
- Supply Ability: 25kg
- Dicyclohexylchlorophosphine
-

- $0.00 / 10Kg
- 2025-12-15
- CAS:16523-54-9
- Min. Order: 1Kg
- Purity: 99%
- Supply Ability: 100kg
- Dicyclohexylchlorophosphine
-

- $1.00 / 1KG
- 2025-12-12
- CAS:16523-54-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 Tons