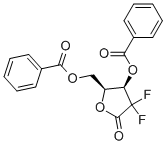
Synthesis of 2-Deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-dibenzoate
Dec 27,2019
2-Deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-dibenzoate (CAS: 122111-01-7) is a compound useful in organic synthesis and it is the key intermediate of gemcitabine [1]. Gemcitabine, a pyrimidine analog, is chemically known as 1-(2'-Deoxy-2', 2'-difluoro-D-ribofuranosyl) -4-aminopyrimidin-2-one. Although Gemcitabine is structurally similar to cytarabine, it has a wider spectrum of antitumour activity due to its different cellular pharmacology and mechanism of action. Gemcitabine is a type of chemotherapy for treating many types of cancers, including lung, pancreatic cancers. It can interfere with the growth of rapidly growing cells like cancer cells and cause cell death [2]. Let see the work mechanism of gemcitabine. gemcitabine acts as a prodrug which is intracellularly phosphorylated to its active diphosphate and triphosphate intermediates. Gemcitabine triphosphate competes with deoxycytidine triphosphate for incorporation into DNA, which results in termination of DNA polymerization. The diphosphate intermediate effectively inhibits ribonucleotide reductase (RRM1) which leads to the depletion of the deoxynucleotide pool and the halt of DNA synthesis.

Scheme 1 Synthesis of 2-Deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-dibenzoate
Gemcitabine is important in clinic medicine, and acts as its key intermediate, 2-Deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-dibenzoate synthesis process has got lot of attention.
Zhou Yong and team synthesized 2-Deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-dibenzoate with N, N-dimethyl-4-aminopyridine as catalyst, and under the reaction of 3R,3S-2-deoxidation-2,2-difluoro-1-Oxo-D-ribofuranose and benzoyl as shown in scheme 1. The product was recrystallized by isopropanol, and diastereoisomer was extracted. In the reaction, the selection of catalysts, reaction temperature and ratio of reactants influence the reaction and yield [3].

Scheme 2 Synthesis of 2-Deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-dibenzoate
As shown in scheme 2, Long Zaihua synthesized 2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate through selective protected the hydroxyl from D-mannital and sodium periodate oxidation, Reformatesky reaction, separating the protective group and cyclization and benzoyl chloride. The process of deprotect group and cyclization and crystallization step were optimized. The results show that the new process, not only can effectively improve the product yield and quality, but also lower the cost and reduce the pollution of the environment [4].
References
[1] Marc A.Labroli, etc., Syntheses of 5′-amino-2′,5′-dideoxy-2′,2′-difluorocytidine derivatives as novel anticancer nucleoside analogs, Tetrahedron Letters, Volume 55, Issue 3, 15 January 2014, Pages 598-602
[2] Ashish Ujagare, etc., An improved one pot process for making key intermediate for gemcitabine HCl, WO2007049295A2.
[3] ZHOU Yong etc., Synthesis and characterization of 3,5-double-O-benzoyl-2 deoxidation-2,2-difluoro-1-Oxo-D-ribofuranose, Yingyong Huagong, 42 (10), 1804-1805, 1807; 2013.
[4] LONG Zai-hua etc., The Synthetic Process Improving of Intermediates for Gemcitabine, Shandong Huagong, 41(12), 80-82; 2012.
- Related articles
- Related Qustion
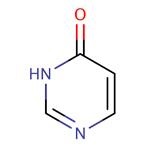
Pyrimidone is the name given to either of two heterocyclic compounds with the formula C4H4N2O: 2-pyrimidone and 4-pyrimidone.....
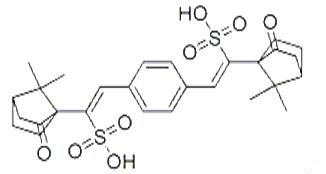
Dec 27,2019Pharmaceutical intermediatesTerephthalylidene dicamphor sulfonic acid (TDSA) is an organic compound and it is a benzylidene camphor derivative. Terephthalylidene dicamphor sulfonic acid is an FDA-approved sunscreen chemical.....
Dec 27,2019Catalyst and AuxiliaryYou may like
2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate manufacturers
- 2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate
-

- $0.00 / 10g
- 2025-12-16
- CAS:122111-01-7
- Min. Order: 10g
- Purity: 97%min
- Supply Ability: 1000g
- 2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate
-

- $0.00 / 25kg
- 2025-11-19
- CAS:122111-01-7
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 2000kg
- 2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate
-

- $15.00 / 1KG
- 2021-07-13
- CAS:122111-01-7
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton