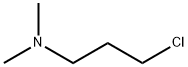
3-Chloro-1-(N,N-dimethyl)propylamine and 3-Chloro-1-(N,N-dimethyl)propylamine Hydrochloride
Dec 12,2025
Introduction
3-Chloro-1-(N,N-dimethyl)propylamine (Figure 1),also known as N,N-dimethyl-3-chloropropylamine or dimethylaminopropyl chloride, is an opalescent turbid liquid with a molecular weight of 121.61 and a boiling point of 130.7°C. It serves as the side chain for the synthesis of numerous pharmaceuticals, including cyclobenzaprine, bendazac, chlorprothixene, chlorpromazine, clomipramine, triflupromazine, imipramine, amitriptyline, and doxepin. These drugs exhibit antipsychotic, antidepressant, and anxiolytic effects, while also demonstrating significant efficacy in anti-inflammation, antipyretic, and analgesic applications.

Synthesis of 3-Chloro-1-(N,N-dimethyl)propylamine
1-Bromine-3-chlorine propane and 33 % dimethylamine water solution were used as the main raw materials, through the reaction of amine alkylation to conduct the technical research of the synthesis of 3-Chloro-1-(N,N-dimethyl)propylamine. By doing the Single-factor test and the Orthogonal test, the optimal synthesis conditions was determined, the raw materials ratio was 1∶1.5, the reaction temperature was 30~35 °C, the reaction time was 4.5 h, the continuous reaction time was 1.5 h, the alkalization pH was 10~11,the total yield was 70.2 %. The experimental method was simple and easy for raw materials, lower costs.[1]
3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride[2]
Use and Exposure
3-Chloro-1-(N,N-dimethyl)propylamine typically exists as its hydrochloride salt,which is a member of the class of nitrogen mustard-type compounds. It is used primarily as an industrial and research organic chemical intermediate acting as an alkylating reagent in Grignard and other types of reactions. It is also used as a pharmaceutical intermediate for the synthesis of many types of drugs, as an agricultural chemical intermediate, as a photographic chemical intermediate, and as a biochemical reagent for enzyme and other studies,such as analgesics, antiarrythmics, antibiotics, anticholesteremics, antidepressants (anxiolytics, tranquilizers),antiischemics, and antineoplastics.
Human occupational or other accidental exposure can occur by inhalation, ingestion, or skin absorption. According to Soper et al., 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride and its analogs are stable compounds and, when used as pharmaceutical intermediates, may persist through drug syntheses and remain as trace contaminants in the final products.
Absorption, distribution, and metabolism
No information on the metabolism of 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride was found in the available literature. Nitrogen mustards are biotransformed to reactive electrophilic aziridinium ions; however, while dialkylaminoethyl chlorides probably exist in equilibrium with cyclic aziridinium ion intermediates at near neutral pH, dialkylaminopropyl chlorides do not.
3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride, a monofunctional nitrogen mustard-type chemical, has been shown tobe a DNA alkylating agent, but weaker than bifunctional nitrogen mustards and without DNA-interstrand crosslinking ability. While higher concentrations of monofunctional nitrogen mustard-type compounds are required for DNA cross-linking than related polyfunctional alkylating agents, exposure of cells to these compounds interferes with the progression of cells from S phase to metaphase. 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride is one of a group of aliphatic and aromatic amine compounds, which act as competitive inhibitors of human kidney histamine N-methyltransferase. 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride, as the free base, inhibited this enzyme by 50% at a concentration of 0.32 mM.
3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride has been studied as one of a group of tertiary amine analogs of choline useful as substrates for investigations of mechanisms of membrane transport. 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride, migrates rapidly across the cell membrane of human erythrocytes(characteristic of lipid-soluble molecules) and, in the protonated form, is a strong inhibitor of choline uptake at the transport carrier site (61% inhibition). An observed decrease in flux ratio (the ratio of infinite-trans to zero-trans flux)indicates a preferential binding by dimethylaminopropyl chloride, hydrochloride at the inner carrier site. 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride has also been demonstrated to have high affinity as an inhibitor of choline uptake (55% inhibition).
Toxicity
Male and female F344/N rats and B6C3F1 mice received 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride (greater than99% pure) in water by gavage for 2 weeks or 3 months. Genetic toxicology studies were conducted in Salmonella typhimurium and mouse peripheral blood erythrocytes.In the 2-week toxicity studies, groups of five male and five female F344/N rats and B6C3F1 mice were administered doses of 0, 6.25, 12.5, 25, 50, or 100 mg 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride/kg body weight in deionized water by gavage, 5 days per week for 16 days. All dosed male and female rats and mice survived until the end of the 2-week study; one vehicle control female mouse died early. Mean body weights of all dosed groups of rats and mice were similar to those of the vehicle control groups. No gross or microscopic lesions were considered related to 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride administration.
In the 3-month toxicity studies, groups of 10 male and 10 female F344/N rats and B6C3F1 mice were administered doses of 0, 6.25, 12.5, 25, 50, or 100 mg/kg in deionized water by gavage, 5 days per week for 3 months. One male rat in the 50 mg/kg group died during week 12 of the study, and one female mouse in the 100 mg/kg group died during week 9 and another during week 13. The final mean body weights of 50 mg/kg male rats and 50 mg/kg female mice were significantly less than those of the vehicle controls. Possible chemical-related clinical findings in rats included lethargy in one 50 mg/kg male and one 100 mg/kg male, tremors in one 100 mg/kg male, and ataxia in one 50 mg/kg male and two 100 mg/kg males. Absolute lung weights in the 25, 50, and 100 mg/kg groups of female mice were significantly less than those of the vehicle controls. Total serum bile acid concentrations were increased in 50 mg/kg male rats and 100 mg/kg male and female rats. The incidence of goblet cell hypertrophy of the nose was significantly increased in 100 mg/kg male rats compared to the vehicle controls. There were no significant histopathologic findings in mice. 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride was mutagenic in the Salmonella typhimurium base substitution strains TA100 and TA1535, with and without hamster or rat liver S9 activation enzymes; no mutagenic activity was seen in TA97 or TA98. No increase in the frequency of micronucleated erythrocytes was seen in peripheral blood of male or female mice administered 3-Chloro-1-(N,N-dimethyl)propylamine hydrochloride for 3 months by gavage.
References
[1] Zhou ZS,et al.Study on the Synthesis of N,N-dimethyl-3-chloropropylamine[J].Guangdong Chemical Industry,2012,39(12):221-222+220.
[2] Abdo K. NTP toxicity studies of dimethylaminopropyl chloride, hydrochloride (CAS No. 5407-04-5) administered by Gavage to F344/N rats and B6C3F1 mice. Toxic Rep Ser. 2007;(75):1-F8.
- Related articles
- Related Qustion
Bis[4-(2-phenyl-2-propyl) phenyl]amine is an important aromatic amine antioxidant,which is used to protect natural rubber and synthetic rubber.....
Dec 12,2025AntioxidantsDiosgenin is a precursor of many steroidal drugs synthesis, an important raw material for artificial synthesis of steroid hormones and steroidal contraceptives.....
Dec 12,2025Biochemical Engineering3-Chloro-1-(N,N-dimethyl)propylamine
109-54-6You may like
3-Chloro-1-(N,N-dimethyl)propylamine manufacturers
- 3-Chloro-1-(N,N-dimethyl)propylamine
-
- $1.10 / 1g
- 2025-11-18
- CAS:109-54-6
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons min
- (3-chloropropyl)dimethylamine
-
- $0.00 / 1KG
- 2022-02-21
- CAS:109-54-6
- Min. Order: 1KG
- Purity: 98.6%
- Supply Ability: 100 tons
- 3-Chloro-1-(N,N-dimethyl)propylamine
-

- $1.00 / 1kg
- 2019-07-06
- CAS:109-54-6
- Min. Order: 1kg
- Purity: 95%-99%
- Supply Ability: as request