Talazoparib (BMN 673): a polymerase inhibitor
Jan 3,2024
Overview
Polymerase (PARP) inhibitors are molecular analogs of ADP ribose, inhibiting the interaction between PARP enzyme and the ADP ribose, and act as a PARP trapping that not only interferes with DNA repair, transcription, and replication but also causes direct lethal DNA double-strand breaks during S-phase by collapse of stalled replication forks. Currently, there are two FDA-approved PARP inhibitors for metastatic breast cancer: talazoparib and olaparib[1]. There are also 3 agents that are still in development: veliparib, niraparib and rucaparib.
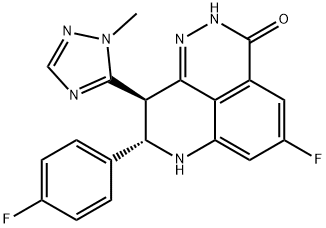
Talazoparib ((8 S,9 R)− 5-fluoro-8-(4-fluorophenyl)− 9-(1-methyl-1 H-1,2,4-triazol-5-yl)− 2,7,8,9-tetrahydro-3 H-pyrido[4,3,2 de]phthalazin-3-one; TLZ; BMN 673) is a PARPi developed by Pfizer and approved in 2018 in USA and 2019 in EU for the treatment of germline BRCA-mutated, HER2-negative breast cancer. Like other PARP inhibitors, talazoparib (formerly known as BMN673) demonstrated in-vitro antitumor activity only in tumors with defects in homologous recombination, mainly BRCA mutations. However, more potent antitumor responses at much lower concentrations were observed when compared to other PARP inhibitors[2].
Synthesis method
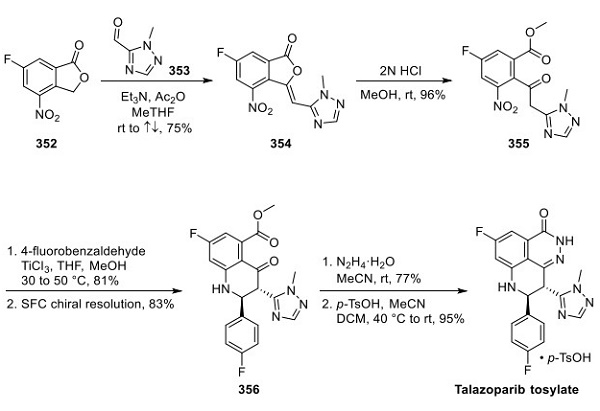
BioMarin has published several routes to talazoparib in the patent literature. The most likely scale route has been exemplified on the kilo scale through enantiopure intermediate 356[3]. Interestingly, the approach to 356 was inspired by a fortuitous byproduct observed during the discovery of talazoparib. Condensation of isobenzofuranone 352 and aldehyde 353 in the presence of triethylamine and acetic anhydride gave rise to compound 354 in 75% yield. Acid-catalyzed the ring opening of lactone 354 with methanol and provided keto-ester 355. Both 354 and 355 were isolated by precipitation and filtration following solvent exchange, avoiding column chromatography. While the mechanistic details of the cascade reaction sequence that converted 355 to 356 has to date not been disclosed, the mechanism presumably proceeds via in situ nitro reduction, imine formation with 4-fluorobenzaldehyde, and Mannich-like intramolecular attack by the proximal triazolylacetic ketone to deliver the racemic heterocycle 356. The trans-relative configuration of ring substituents was the major product obtained, which could be isolated by crystallization in 81% yield. Chiral separation was reported on kiloscale using either SFC (83% yield, 97% purity) or simulated moving bed (SMB) (productivity greater than 6 (kg feed/day)/kg chiral stationary phase). The initial route relied on chiral separation via SFC on talazoparib itself; however, owing to the improved solubility of the racemate 356 compared to the API, chiral separation of racemic 356 was found to improve separation efficiency and scalability. Scientists from Guangzhou Wellhealth Biopharmaceutical Co. have reported an alternative resolution of talazoparib on a smaller scale that avoids chiral separation, instead using D-(−)-tartaric acid to isolate a late-stage intermediate. Treatment of 356 with hydrazine monohydrate secured the tricyclic core in 77% yield, which was then converted to the tosylate salt to give talazoparib (shown above).
References
[1] Pedro Exman, Sara M Tolaney, Romualdo Barroso-Sousa. “Evidence to date: talazoparib in the treatment of breast cancer.” OncoTargets and therapy (2019): 5177–5187.
[2] Alejandro Mateos-Pujante, Inmaculada Andreu, M Consuelo Jiménez. “Assessment of the PARP inhibitor talazoparib photosafety profile.” Biomedicine & Pharmacotherapy 167 (2023): 115593.
[3] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringOmidenepag isopropyl (OMDI), administered as a 0.002% ophthalmic solution, was approved in Japan in 2018 for treating glaucoma and ocular hypertension.....
Jan 3,2024Biochemical EngineeringBMN 673
1207456-01-6You may like
- Talazoparib
-
- $35.00 / 2mg
- 2025-12-12
- CAS:1207456-01-6
- Min. Order:
- Purity: 99.92%
- Supply Ability: 10g
- BMN673/Talazoparib
-

- $0.00/ kg
- 2024-11-26
- CAS:1207456-01-6
- Min. Order: 1kg
- Purity: 99%, Single impurity<0.1
- Supply Ability: 1 ton
- Talazoparib
-

- $15.00 / 1KG
- 2021-07-13
- CAS:1207456-01-6
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton