The biological activity of Piperonylic acid
May 20,2025
Introduction
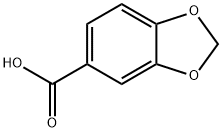
Piperonylic acid (Figure 1) is a natural molecule extracted from the bark of the Paracoto tree that roughly fulfills all of these requirements. Piperonylic acid contains a methylenedioxyphenyl (MDP) function at a position suitable for oxidative attack by CYP73A1. Many compounds with methylenedioxyphenyl function have been shown to inhibit mammalian or insect P450 enzymes both in vitro and in vivo. They were shown to act as mechanism-based inactivators and to require P450-catalyzed metabolism to generate a metabolic intermediate forming a stable complex with the enzyme. Available data suggest that the metabolic intermediate (MI) is likely a carbene that binds as the sixth coordinant to the heme iron.

Biological activity
Selectivity of PA toward C4H
The efficiency of piperonylic acid as a mechanism-based inhibitor of trans-cinnamate 4-hydroxylase (C4H) seems to largely rely on its high structural homology to cinnamic acid. However, the MDP function can potentially inactivate any P450 enzyme. To determine if piperonylic acid was selective for C4H or if it also inactivated other P450s, researchers made an estimation of the proportion of P450 converted into the MI complex and compared it with the proportion of C4H in total plant microsomes. No piperonylic acid metabolite was detected by HPLC analysis of the incubation medium,suggesting that no metabolite leaves the active site of the P450(s) that catalyzes its activation and binds other P450 enzymes. The amount of P450-MI complex determined by spectrophotometry can thus be used to measure the proportion of total P450 capable of metabolizing piperonylic acid in a mixture such as that in plant microsomes. △A427-490 was proportional to the CYP73A1 content of the incubation medium: the ε427-490 was 102 mm-1 cm-1. This extinction coefficient was then used to determine the P450-MI content in microsomes of Jerusalem artichoke tubers treated to modify P450enzyme subpopulations. CYP73A1 contents were determined in the same microsomes from the substrate-binding spectra. The difference spectra observed upon incubation of plant microsomes with PA and NADPH were identical to those obtained with recombinant CYP73A1. Only the results obtained with microsomes prepared from aminopyrine-treated tissues suggested the possible existence of another P450 subspecies able to form a MI complex with piperonylic acid.[1]
Activation of EGFR and Wnt/β-Catenin Pathway
Piperonylic acid is a small molecule that induces EGFR activation in keratinocytes. However, the effects of piperonylic acid on dermal papilla cells (DPCs) in regard to the stimulation of hair growth have not been studied. In the present study, piperonylic acid was shown to activate the Wnt/β-catenin signaling pathway in addition to the EGFR signaling pathway in DPCs. Piperonylic acid suppressed DKK1 expression, which presumably promoted the accumulation of β-catenin in the nucleus. In addition, piperonylic acid promoted cyclin D upregulation and cell growth and increased the expression of alkaline phosphatase (ALP), a DPC marker. In a clinical study, the group that applied a formulation containing piperonylic acid had a significantly higher number of hairs per unit area than the placebo group. These results identify piperonylic acid as a promising new candidate for hair loss treatment.[2]
Alter growth and reactive oxygen species-scavenging capacity
p-Coumaric acid synthesis in plants involves the conversion of phenylalanine to trans-cinnamic acid via phenylalanine ammonia-lyase (PAL), which is then hydroxylated at the para-position under the action of trans-cinnamic acid 4-hydroxylase. Alternatively, some PAL enzymes accept tyrosine as an alternative substrate and convert tyrosine directly to p-coumaric acid without the intermediary of trans-cinnamic acid. In recent years, the contrasting roles of p-coumaric acid in regulating the growth and development of plants have been well-documented. To understand the contribution of trans-cinnamic acid 4-hydroxylase activity in p-coumaric acid-mediated plant growth, mineral content accumulation and the regulation of reactive oxygen species (ROS), we investigated the effect of piperonylic acid (a trans-cinnamic acid 4-hydroxylase inhibitor) on plant growth, essential macroelements, osmolyte content, ROS-induced oxidative damage, antioxidant enzyme activities and phytohormone levels in chia seedlings. Piperonylic acid restricted chia seedling growth by reducing shoot length, fresh weight, leaf area measurements and p-coumaric acid content. Apart from sodium, piperonylic acid significantly reduced the accumulation of other essential macroelements (such as K, P, Ca and Mg) relative to the untreated control. Enhanced proline, superoxide, hydrogen peroxide and malondialdehyde contents were observed. The inhibition of trans-cinnamic acid 4-hydroxylase activity significantly increased the enzymatic activities of ROS-scavenging enzymes such as superoxide dismutase, ascorbate peroxidase, catalase and guaiacol peroxidase. In addition, piperonylic acid caused a reduction in indole-3-acetic acid and salicylic acid content. In conclusion, the reduction in chia seedling growth in response to piperonylic acid may be attributed to a reduction in p-coumaric acid content coupled with elevated ROS-induced oxidative damage, and restricted mineral and phytohormone (indole-3-acetic acid and salicylic) levels.[3]
Inhibitory effects on the excessive proliferation
In this study,combining in vivo and in vitro experiments, researchers explored the function and mechanism of piperonylic acid inhibiteing excessive proliferation of vascular smooth muscle cells, and intimal hyperplasia and luminal stenosis after blood vessel injury. A model of rat thoracic aorta restenosis after balloon injury was constructed, intragastrically administered with piperonylic acid. 21 days later, their thoracic aortas were subjected tests of morphology, SM-α-actin, proliferating cell nuclear antigen (PCNA), and expression levels of P21 and P27 by HE staining, immunohistochemistry, and computer image analysis. The proliferation of vascular smooth muscle cells induced by fetal calf serum was active, and the expressions of P21 and P27 were low. Piperonylic acid obviously promoted the protein expressions of P21 and P27 while inhibiting proliferation and DNA synthesis. After the injury of rat thoracic aorta, the cells moved towards the intima and proliferated excessively, leading to evident neogenesis of intima and luminal stenosis. The SM-α-actin immunohistochemistry confirms that the intima contained abundant smooth muscle cells and that the expression of PCNA was high while the expression of P21 and P27 was low. The intervention of piperonylic acid significantly facilitated the gene expressions of P21 and P27, lowered the PCNA expression, and inhibited the formation of intima and the reconstruction of pathological vessels, thus remarkably suppressing luminal stenosis. Piperonylic acid can inhibit the excessive proliferation of vascular smooth muscle cells and the lumen narrowing after injury of blood vessels, the mechanism of which is associated with the promoted gene expressions of cell cycle key regulators P21 and P27.[4]
References
[1]Schalk M, Cabello-Hurtado F, Pierrel MA, Atanossova R, Saindrenan P, Werck-Reichhart D. Piperonylic acid, a selective, mechanism-based inactivator of the trans-cinnamate 4-hydroxylase: A new tool to control the flux of metabolites in the phenylpropanoid pathway. Plant Physiol. 1998;118(1):209-218. doi:10.1104/pp.118.1.209
[2]Han SH, Jo KW, Kim Y, Kim KT. Piperonylic Acid Promotes Hair Growth by Activation of EGFR and Wnt/β-Catenin Pathway. Int J Mol Sci. 2024;25(19):10774. Published 2024 Oct 7. doi:10.3390/ijms251910774
[3]Nkomo M, Gokul A, Ndimba R, Badiwe M, Keyster M, Klein A. Piperonylic acid alters growth, mineral content accumulation and reactive oxygen species-scavenging capacity in chia seedlings. AoB Plants. 2022;14(3):plac025. Published 2022 May 26. doi:10.1093/aobpla/plac025
[4]Lin L, Hong T. Inhibitory effects of piperonylic acid on the excessive proliferation of vascular smooth muscle cells and luminal stenosis. Bratisl Lek Listy. 2014;115(12):761-765. doi:10.4149/bll_2014_147
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering2′-Deoxyadenosine has extraordinary strong potentials as a intermediate compound of anti-cancer and anti-viral nucleoside drugs. Synthesis method is reported.....
May 20,2025Amino Acids and DerivativesPiperonylic acid
94-53-1You may like
Piperonylic acid manufacturers
- Piperonylic acid
-

- $0.00 / 25Kg/Drum
- 2025-12-18
- CAS:94-53-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200mt/year
- Piperonylic acid
-

- $10.00 / 1KG
- 2025-12-11
- CAS:94-53-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- Piperonylic acid
-
- $0.00 / 10mg
- 2025-09-25
- CAS:94-53-1
- Min. Order: 10mg
- Purity: 0.98
- Supply Ability: 10g