The introduction of bis(3',5')-cyclic diguanylic acid
Jan 19,2023
General description
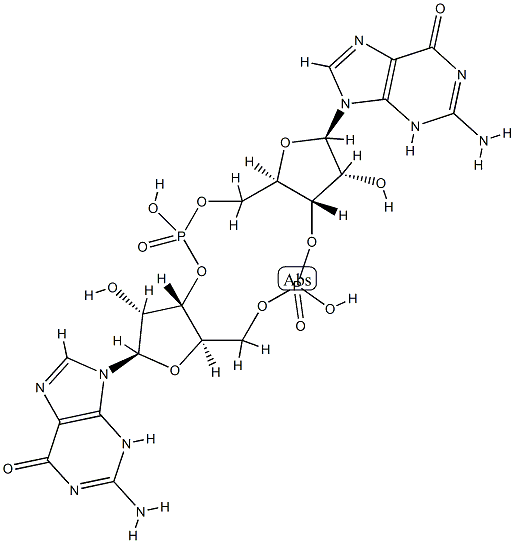
The bis(3',5')-cyclic diguanylic acid, with the CAS No: 61093-23-0, is also known as Cyclic diguanosine monophosphate (c-diGMP/c-di-GMP). This chemical’s molecular formula is C20H24N10O14P2 and molecular weight is 690.41. Bis(3',5')-cyclic diguanylic acid (c-di-GMP) is a naturally occurring small cyclic dinucleotide found in bacteria. It was first identified as an allosteric regulator of cellulose synthase in Gluconacetobacter xylinus (formerly Acetobacter xylinum) [1]. It is now recognized as a universal bacterial second messenger. It displays a complex polymorphism in solution, existing under an equilibrium of monomer, dimer, tetramer, and octamers. Some of these assemblies, particularly dimers, have been found to play important roles in biological processes. Its structure is as follows:
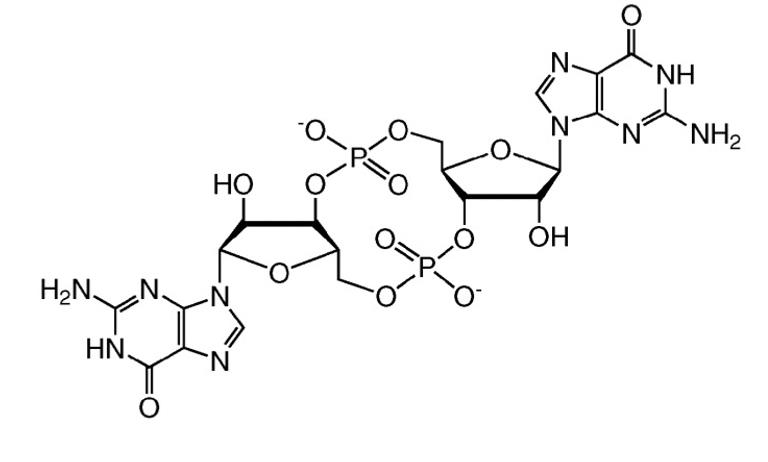
Figure 1 Structure of c-di-GMP.
Biosynthesis and degradation of c-di-GMP
Cellular levels of c-di-GMP vary in bacterial species and in different phases of bacterial growth [2]. For example, based on mass spectrometric results, it was estimated that there are between 86 and 255 c-di-GMP molecules per cell in Samonella typhimurium strain UMR1 after 10 to 24 h of growth [2]. It is recognized that cellular c-di-GMP concentration is regulated by two enzymes, diguanylate cyclases (DGC) and phospho- diesterases (PDE) (Figure 2) [3]. DGC utilizes guanosine triphosphate (GTP) as substrate and catalyses the formation of c-di-GMP through a linear intermediate pppGpG. This enzyme contains a conserved GGDEF (Gly–Gly–Asp–Glu–Phe) domain. c-di-GMP can also bind to some DGC proteins, resulting in repression of DGC activity via allosteric changes. PDE degrades c-di-GMP into guanosine mono- phosphate (GMP) through a linear intermediate pGpG. The latter enzyme features a conserved EAL domain (a domain enriched in Glu–Ala–Leu). In some bacteria, the HD-GYP domain (His–Asp, Gly–Tyr–Pro, a subgroup of the HD super- family of metal-dependent phosphohydrolases containing an additional GYP motif) replaces the EAL domain [3]. The GGDEF domain was first identified in PleD in the aquatic bacterium Caulobacter crescentus, which is involved in the transition between motile and flagellum-containing swarmer cells to cells in an attached phase of the life cycle. PleD has been confirmed to possess cyclase activities. In response to first messenger stimulation, cellular c-di-GMP concentration is altered through the DGC and/or PDE activities. Binding of c-di-GMP to downstream proteins causes allosteric changes to the proteins, leading to the manifestations of certain cellular functions and/or phenotypic transitions. c-di-GMP can also inhibit DGC activity.
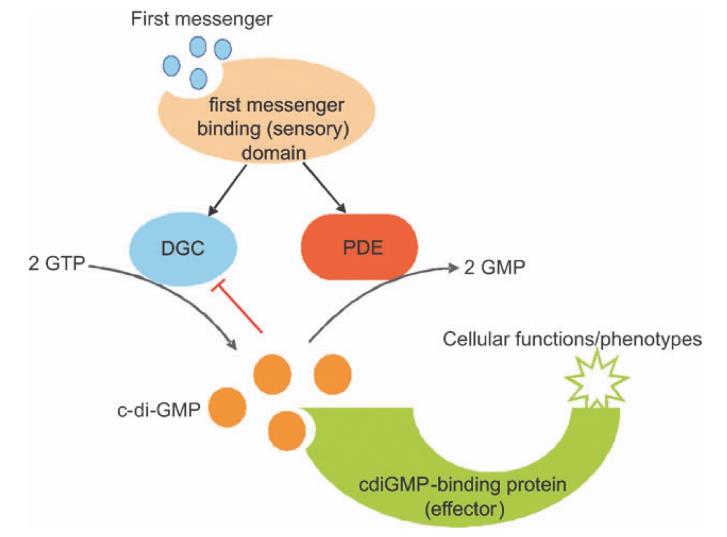
Figure 2 c-di-GMP synthesis, degradation and signalling.
Chemical and enzymatic synthesis of c-di-GMP and analogues
The chemical synthesis of c-di-GMP encompasses two challenges, choice of phosphorylation method and protection of 20-OH. These considerations are of importance if larger quantities of c-di-GMP samples are to be required for biological investigation. So far, c-di-GMP syntheses have been carried out mostly in solution using the phosphotriester, phosphoramidite, H-phosphonate, or the modified H-phosphonate approaches. The first chemical synthesis of c-di-GMP was reported by van Boom et al [4]. via the phosphotriester approach, where the phosphotriester chemistry was used for phosphorylation and tetrahydropyran-2-yl (Thp) was used to block 20-OH. In the key cyclization step (1a to 2a, Figure 3), a 74% yield was achieved. Hayakawa et al. later on utilised a modified phospho- triester chemistry to affect the cyclization [5]. In this synthesis 20-OH was protected by t-butyldimethylsilyl (TBDMS) group. Allyl, instead of 2-chlorophenyl-, phosphotriester was used in the cyclization reaction to give the fully-protected c-di-GMP in 75% yield (1b to 2b in Figure 3). Subsequent deprotection gave c-di-GMP in 77% yield after purification by preparative HPLC. The same lab subsequently executed an improved method for c-di-GMP synthesis, using another modified phosphotriester approach (1c to 2c in Figure 3) [6]. HPLC purification of the fully-deprotected c-di-GMP was necessary to give c-di-GMP in 89% yield.
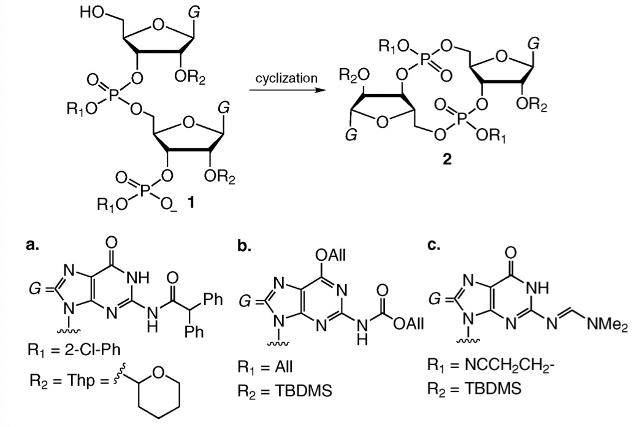
Figure 3 Cyclization using the phosphotriester chemistry. Different triesters and 20-OH protecting groups were used.
Biological function of c-di-GMP
Among the characterised c-di-GMP-mediated signalling networks, only the pathways involving c-di-GMP-binding proteins are affected by the binding events. This contrasts the widespread of GGDEF and EAL domains in bacteria, which suggests a network of pathways involving c-di-GMP in global cellular functions. It was revealed for the first time that c-di-GMP in many bacteria is sensed by a riboswitch in mRNA that controls the expression of genes involved in numerous fundamental cellular processes, such as virulence gene expression, pilus formation, and flagellum biosynthesis [7]. Although the transcriptional cascades that trigger these changes are indeed very complex, c-di-GMP has been shown to play a very important role in adhesion, cell-to-cell communica- tion, exopolysaccharide formation, and motility [8]. For many bacterial species, c-di-GMP acts as a ‘‘lifestyle switch regulator’’ in regulating the transition between sessile and motile lifestyles. One aspect of c-di-GMP properties that has attracted significant interest among immunologists is its extraordinary immunostimulatory and immunomodulatory properties and its potential application as a vaccine adjuvant. In the first such studies, c-di-GMP was shown to induce the activation and maturation of human immature dendritic cells (DCs), professional antigen-presenting cells, in vitro [9].
References
[1]P. Ross, H. Weinhouse, Y. Aloni, D. Michaeli, P. Weinbergerohana, R. Mayer, S. Braun, E. Devroom, G. A. Vandermarel, J. H. van Boom and M. Benziman, Nature, 1987, 325, 279–281.
[2]R. Simm, M. Morr, U. Rerriminghorst, M. Andersson and U. Romling, Anal. Biochem., 2009, 386, 53–58.
[3]M. Y. Galperin, A. N. Nikolskaya and E. V. Koonin, FEMS Microbiol. Lett., 2001, 203, 11–21.
[4]P. Ross, R. Mayer, H. Weinhouse, D. Amikam, Y. Huggirat, M. Benziman, E. Devroom, A. Fidder, P. Depaus, L. Sliedregt, G. A. Vandermarel and J. H. van Boom, J. Biol. Chem., 1990, 265, 18933–18943.
[5]Y. Hayakawa, R. Nagata, A. Hirata, M. Hyodo and R. Kawai,Tetrahedron, 2003, 59, 6465–6471.
[6]M. Hyodo and Y. Hayakawa, Bull. Chem. Soc. Jpn., 2004, 77, 2089–2093.
[7]N. Sudarsan, E. R. Lee, Z. Weinberg, R. H. Moy, J. N. Kim, K. H. Link and R. R. Breaker, Science, 2008, 321, 411–413.
[8]A. D. Tischler and A. Camilli, Mol. Microbiol., 2004, 53, 857–869.
[9]D. K. R. Karaolis, T. K. Means, D. Yang, M. Takahashi, T. Yoshimura, E. Muraille, D. Philpott, J. T. Schroeder, M. Hyodo, Y. Hayakawa, B. G. Talbot, E. Brouillette and F. Malouin, J. Immunol., 2007, 178, 2171–2181.
- Related articles
- Related Qustion
- Bis(3',5')-cyclic diguanylic Acid: Physiological Activities, Synthesis and Degradation Apr 17, 2024
Bis(3',5')-cyclic diguanylic acid regulates bacterial and eukaryotic processes, synthesized by cyclases and degraded by phosphodiesterases.
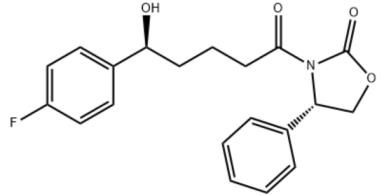
The (4S)-3-[(5R)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-pheny-1,3-oxazolidin-2-one,?is an chiral intermediate in the synthesis of chiral side chain of ezetimibe.....
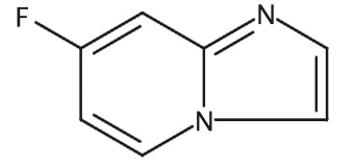
Jan 18,2023APIThe 7-Fluoro-imidazo[1,2-a]pyridine, with the CAS No: 1260903-17-0, is also known as Imidazo[1,2-a]pyridine, 7-fluoro-. This chemical’s molecular formula is C7H5FN2.....
Jan 19,2023APIbis(3',5')-cyclic diguanylic acid
61093-23-0You may like
bis(3',5')-cyclic diguanylic acid manufacturers
- Cyclic-Di-GMP, Sodium Salt
-
- $0.00 / 1kg
- 2025-12-15
- CAS:61093-23-0
- Min. Order: 1kg
- Purity: ≥95.0%
- Supply Ability: 1000 kg
- Cyclic-di-GMP
-

- $2500.00 / 100mg
- 2025-08-21
- CAS:61093-23-0
- Min. Order:
- Purity:
- Supply Ability: 10g
- c-di-GMP; cyclic diguanylate;bis(3',5')-cyclic diguanylic acid
-
- $0.00 / 50g
- 2025-08-21
- CAS:61093-23-0
- Min. Order: 10g
- Purity: 99% HPLC
- Supply Ability: 10000