The relationship between Xanthine and physiological activities
Dec 27,2023
Description
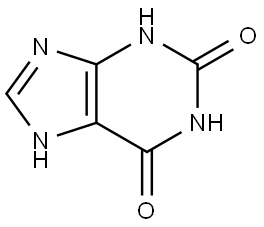
In chemical nomenclature, xanthine is 3, 7-dihydro-purine-2, 6-dione. In animal tissue, xanthine is derived from guanine and adenosine-3-phosphate [1] by catabolic reactions in the body.
Structure
The structure of xanthine consists of two fused rings: one ring is six-membered, and the other is five-membered [1]. Theoretically, two types of tautomerism are displayed in the xanthine molecule. The first is annular i.e. migration of proton of imidazole ring between N7 and N9 positions. Second is lactim-lactam i.e. migration of proton between N1 and N3 and oxygen of carbonyl group at C2 position. Despite annular tautomerism in xanthine, 7H form predominates over 9H formation.
Physiological activity
An increase in xanthine concentration may be a symptom of several physiological diseases like a depressed purine salvage pathway, which leads to Lesch-Nyhan syndrome. Xanthinuria may develop due to decreased xanthine oxidase activity[2]. Unrestrained xanthinuria leads to diseases like stone formation in the kidneys, muscle disease, and urinary tract ailments. Xanthine is excreted 10 -times more swiftly than uric acid, but its concentration is low in urine because xanthine is changed into uric acid by XOD. Due to the low solubility of xanthine, increased xanthine concentration can lead to renal calculus disease. The level of xanthine in human urine varies between 41 and 161 mM. Xanthine concentration in serum is 100 times lower than uric acid. After the death of an organism, degradation of nucleotides to hypoxanthine and xanthine occurs. Techniques used in determining xanthine provide information to assess the quality & conditions required for storing meat products and, in pharmaceutical industries, for making a good variety of products.
References
[1] Ahlawat, Jyoti Minakshi Sharma and Chandra S. Pundir. “Advances in xanthine biosensors and sensors: A review.” Enzyme and Microbial Technology 69 1 (2023).
[2] Nivedita Singh. “Xanthine scaffold: scope and potential in drug development.” Heliyon 4 10 (2018): e00829.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringDimethyl Sulfoxide (DMSO) is a polar aprotic molecule that has the peculiar property of having a mildly nucleophilic sulfur and a basic oxygen. It is used as a reagent in a variety of different reactions.....
Dec 27,2023Organic ChemistryXanthine
69-89-6You may like
- Diosgenin:Uses,Functions and Synthesis
Dec 12, 2025
- Biosynthesis of Cyclopamine from Cholesterol
Dec 10, 2025
- Synthesis of ribociclib
Dec 10, 2025
- 2,6-Dihydroxypurine (Xanthine)
-

- $0.00 / 1Kg/Bag
- 2025-12-13
- CAS:69-89-6
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 3500kg/month
- Xanthine
-

- $0.00 / 1KG
- 2025-12-12
- CAS:69-89-6
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 10MT
- 2,6-Dihydroxypurine
-

- $9.90 / 1KG
- 2025-12-11
- CAS:69-89-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5tons