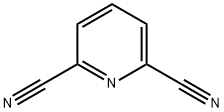
the synthesis of 2,6-Pyridinedicarbonitrile
Dec 27,2019
2,6-Pyridinedicarbonitrile is a heterocyclic dinitrile, which may be used to synthesize bis-tetrazoles and pyridine-based tridentate ligand 2,6-bis(α-aminoisopropyl)pyridine.It is also an important organic intermediate to synthetize substituted pyridine products.
The following example is about its application on the synthesis of light-emitting elements [1]

First, 15.4 g (119 mmol) of 2,6-pyridinedicarbonitrile and 250 mL of methanol(dehydrated) were put into a 500-mL three-neck flask and mixed. Then, 591 mg (10.9 mmol) of sodium methoxide was added to this mixture, and the mixture was stirred under a nitrogen stream at room temperature for 16 hours. After that, 12.7 g (238 mmol) of ammonium chloride was added, and the mixture was stirred under a nitrogen stream at room temperature for two days. After the reaction, the reaction mixture was concentrated, and ethyl acetate was added thereto. The mixture was irradiated with ultrasonic waves, and the lump was crushed into pieces and then suction filtered to give 25.5 g of a white solid in a yield of 91 percent.
The following example is about its application on the synthesis of bis(amidoxime)s [2]

General procedure: A 50wtpercent solution of hydroxylamine in water (8.0mmol, 4 equiv., 2 equiv./nitrile function) was added to a solution of dinitrile (2.0mmol, 1 equiv.) in ethanol (10mL), and the solution was stirred for 20h at room temperature. The solvent was evaporated under vacuum. The resulting compound was either chromatographed on silica gel or washed with an appropriate solvent and recrystallized.
The following example is about its application on the synthesis of macrocycles containing both tetrazole and pyridine functionalities [3]

A suspension of 2,6-pyridinedicarbonitrile (2.57 g, 20 mmol), sodium azide (2.86 g, 43 mmol), ammonium chloride (2.35 g, 43 mmol) and lithium chloride (0.6 g, 14 mmol) in anhydrous dimethylformamide (60 mL) was stirred for 10 h at 110 °C. After this time, the solution was cooled and the insoluble salts were removed by filtration. The solvent was then evaporated under reduced pressure and the residue was dissolved in deionised water (200 mL) and acidified with concentrated HCl (3 mL), to initiate precipitation. The product was filtered, washed with water (3×40 mL) and dried to give 2,6-di(2H-tetrazol-5-yl)pyridine (2) as a white crystalline powder. Purification by recrystallisation from methanol to give white needles (3.08 g, 14.3 mmol, 72 percent);
The following example is about its application on the synthesis of 1-aziridino-1-hydroxyiminomethyl-derivates [4]

To a solution of hydroxylamine hydrochloride (18.07 g; 26 mmol) and NaOH (10.40 g; 26 mmol) in H2O (90 ml) is added dropwise with vigorous stirring a solution of pyridine-2,6-dicarbonitrile (12.9 g; 10 mmol) in ethanol (60 ml). An exothermic reaction occurs, and stirring is then continued for 1.5 h at 40-50° C. After cooling, the precipitate is filtered off and washed with H2O. Obtained after drying is 16.5 g (85 percent of the theoretical) of product.
References
1.Semiconductor Energy Laboratory Co., Ltd. Yamada Y, Inoue H, Suzuki T, Seo S. Organic compound, light-emitting element, display module, lighting module, light-emitting device, display device, electronic device, and lighting device. WO2016/132250[P], 2016, A1, Paragraph 0259; 0260.
2.Stemper J, Tuo W, Mazarío E, Helal AS, Djurovic A, Lion C, El HCJM, Maurel F, Hémadi M, Le GT. Synthesis of bis(amidoxime)s and evaluation of their properties as uranyl-complexing agents[J]. Tetrahedron, 2018, 74(21):2641-2649.
3.Fleming A, Gaire J, Kelleher F, McGinley J, McKee V. Synthesis and characterization of macrocycles containing both tetrazole and pyridine functionalities[J]. Tetrahedron, 2011, 67(18):3260-3266.
4.Kalvins I. 1-aziridino-1-hydroxyiminomethyl-derivates, method for the production thereof and medicaments containing said compounds. US7078418[P], 2006, B1, Page column 5-6.
- Related articles
- Related Qustion
Bromodiphenylmethane is a halogenated building block. Benzhydryl group was preferred to the commoner benzyl to protect 2-nitrophenol in the Bartoli (vinyl Grignard) synthesis of 7-hydroxyindole. Protection was by reaction with the phenol in....
Dec 27,2019Organic ChemistryTetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vit....
Dec 27,2019Chemical Reagents2,6-Pyridinedicarbonitrile
2893-33-6You may like
2,6-Pyridinedicarbonitrile manufacturers
- 2,6-Dicyanopyridine
-

- $0.00 / 1kg
- 2024-02-26
- CAS:2893-33-6
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: Kgs
- 2,6-Pyridinedicarbonitrile
-
- $1.00 / 1KG
- 2020-01-10
- CAS:2893-33-6
- Min. Order: 1KG
- Purity: ≥98%
- Supply Ability: 20 tons