Tiglic Acid and Its Derivatives: Coordination Compounds, Isolation, Bioactivities and Photoisomerization Kinetics
May 9,2025
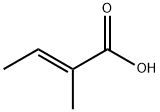
Tiglic acid is a compound found in croton oil and certain beetle secretions, with a systematic name of trans-1,2-dimethylacrylic acid. It is structurally related to acrylic acid and its esters are not polymerizable.

New Dinuclear Copper Coordination Compound with Tiglic Acid
The first coordination compound of copper and tiglic acid named tetrakis(μ-tiglato)bis(tiglic acid)dicopper(II) was synthesized and crystallized from water solution. Its structure was determined and analyzed based on X-ray diffraction measurement. The paddle-wheel coordination system of the investigated compound was compared with other similar copper structures known in the literature. The Hirshfeld analysis was used for the detailed analysis of intermolecular interaction. The new compound was also characterized in terms of infrared absorption, thermal, and magnetic properties. The antiferromagnetic coupling of copper ions was found. More commonly known as tiglic acid, (2E)-2-Methylbut-2-enoic acid is one of the simplest unsaturated monocarboxylic acids. It is a volatile, crystalline solid with a distinctive, sweet odor. Tiglic acid naturally occurs in croton oil. It can also be found in the secretions of certain species of beetles. Along with angelic acid, it forms a pair of cis–trans isomers. Unsaturated organic acids are important compounds considering their industry applications. Their esters are widely used in the food, cosmetic, and pharmaceutical industries. Tiglic acid is not an exception—along with its derivatives, it is an important flavoring agent and fragrance additive. Tiglic acid can be used in the processes of manufacturing rum, caramel, bread, and fruit essences.[1]
Its derivatives also exhibit potential anti-inflammatory and antiproliferative activity. One of the biggest areas of interest concerning tiglic acid is its biosynthesis—naturally occurring flavors and fragrances synthesized using enzymes can be labeled as “natural”. The coordination chemistry of tiglic acid has not been widely explored to date. In the Cambridge Structural Database (CSD), there are only 15 compounds whose structures contain such acid or its anion; thus, every new research effort in this field provides important knowledge and fills the existing literature gap. The studied compound tetrakis(μ-tiglato)bis(tiglic acid)dicopper(II) is the first coordination compound of copper with tiglate anion, the structure of which was determined. This is a dinuclear compound, whose two copper cations are bridged by four tiglate anions with syn–syn mode, forming a paddle-wheel structure. The coordination sphere of the central atom is completed by monodentate tiglic acid coordinating by carbonyl oxygen.
The reaction between copper(II) carbonate hydroxide and tiglic acid led to obtaining the dinuclear copper compound of the paddle-wheel structure composed of four syn–syn bridging tiglate anions and two monodentate tiglic acid molecules. The structural data supported by the bond valence theory proves that interaction between copper cations in [Cu2(tig)4(tigH)2] has a nonbonding character. The OH group of tiglic acid forms an intramolecular hydrogen bond with carboxylate oxygen of one tiglate anion. The involvement of one tiglate oxygen in h-bond leads to significant lengthening of its coordination bond with copper in comparison to the rest of the tiglate oxygens. The supramolecular structure of the studied compound is stabilized by H•••H dispersive interactions and weak C-H•••O and C-H•••C hydrogen bonds. The FT-IR spectrum contains bands corresponding to both tiglic acid and tiglate anion. The vibration modes of carboxylic and carboxylate groups are well distinguishable. Thermal analysis showed that tiglic acid molecules decompose before tiglate anions. The final product of decomposition is CuO.
Hydroxylated Ethacrylic and Tiglic Acid Derivatives
Two new hydroxylated ethacrylic acid derivatives (compounds 1 and 2) and 11 new hydroxylated tiglic acid derivatives (compounds 3–13), together with one known compound (compound 14), were isolated from the stems and branches of Enkianthus chinensis. Their structures were established by extensive spectroscopic analyses, while their absolute configurations were determined by X-ray crystallographic methods (compounds 1 and 2), Mo2(OAc)4-induced electronic circular dichroism experiments (compounds 3 and 4), and chemical methods (compounds 5–11). This study is the first investigation on the secondary metabolites of this species. The anti-inflammatory activities of all isolated compounds were evaluated in an LPS-induced mouse peritoneal macrophage model. Notably, compounds 3 and 12 both exerted potent inhibitory effects on NO production with IC50 values of 2.9 and 1.2 μM, respectively.[2]
Ethacrylic and tiglic acids, which are five-carbon unsaturated carboxylic acids, have been found to occur extensively in plants and insects. These two acids are defensive chemical constituents in insects,while some volatile tiglic acid esters are important flavor compounds in plants. Ethacrylic and tiglic acids usually occur in the form of esters with terpenoids, alkaloids, resin glycosides, and phenols. Numerous studies have showed that their derivatives possess significant pharmacological activities, including cytotoxic and antiviral activities. The first tiglic acid derivative to be isolated from a plant in the genus Enkianthus was methyl 4,5-dihydroxytiglate cinnamate, which exhibited an antifungal inhibitory effect on Cochliobolus miyabeanus.Enkianthus chinensis Franch. is regarded as a poisonous plant, and an extract has been reported to treat phlebitis in a clinical setting. However, there has been no chemical investigation concerning the secondary metabolites of this species. To investigate the bioactive components of E. chinensis, a chemical study of the stems and branches was carried out for the first time. As a result, 13 new hydroxylated ethacrylic and tiglic acid derivatives, enkianthuins A–M (1–13), along with one known compound, methyl 4,5-dihydroxytiglate (14), were isolated. The structures of 1–13 were determined by spectroscopic analysis and by single-crystal X-ray crystallography, as well as chemical hydrolysis. Herein, the isolation, structure elucidation, and potential anti-inflammatory activities of these isolated compounds are described in detail.
Kinetic Study of the Photoreactions of Two Zn-Coordinated Tiglic Acid Molecules
[Zn(TA)2(H2O)2] (H-TA=tiglic acid) has been embedded in a framework composed of CECR (CECR=C-ethylcalix[4]resorcinarene) molecules to examine its E→Z photoisomerization in a periodic framework. The photoisomerization of tiglic acid in CECR-[Zn(TA)2(H2O)2]⋅4 H2O proceeds without the [2+2]-dimerization reaction that often occurs in crystals of uncomplexed analogues, and without breakdown of the crystal lattice that frequently occurs in neat crystals. The two Zn-coordinated TA molecules are located in different size cavities. The rate constants of the isomerization reaction are strongly affected by the size of the reaction cavity. Analysis of the temperature dependence of the reaction rates and the occupancies in the final photostationary state shows that the activation energies and the standard enthalpies of activation are dependent on the difference between the reaction cavities. This is the first quantitative diffraction study of solid-state E/Z isomerization of a metal-coordinated ligand in a periodic host environment.[3]
We conclude that supramolecular photocrystallography allows quantitative analysis of solid state reactions which in neat crystals are likely to lead to crystal breakdown. The analysis of the SCSC E→Z photoisomerization of Zn-coordinated tiglic acid shows a pronounced effect of the crystalline environment on the kinetics of the isomerization. The analysis described here and related studies reported elsewhere indicate the potential of supramolecular frameworks to enhance our understanding of photochemical processes in molecular crystals. Martinez has calculated a fs-scale conversion rate for the isolated ethylene molecule. The reaction in the solid state are obviously much slower, and their rate can be tuned by changing the temperature, which implies that the intermediate state may be investigated by time-resolved diffraction methods.
References
[1]Świątkowski M, Lanka S, Czylkowska A, Gas K, Sawicki M. Structural, Spectroscopic, Thermal, and Magnetic Properties of a New Dinuclear Copper Coordination Compound with Tiglic Acid. Materials (Basel). 2021 Apr 23;14(9):2148. doi: 10.3390/ma14092148. PMID: 33922582; PMCID: PMC8122876.
[2]Wang, Hai-Qiang et al. “Hydroxylated Ethacrylic and Tiglic Acid Derivatives from the Stems and Branches of Enkianthus chinensis and Their Potential Anti-inflammatory Activities.” Journal of natural products vol. 83,10 (2020): 2867-2876. doi:10.1021/acs.jnatprod.0c00286
[3]Zheng, Shao-Liang et al. “Supramolecular solids as a medium for single-crystal-to-single-crystal E/Z photoisomerization: kinetic study of the photoreactions of two Zn-coordinated tiglic acid molecules.” Chemistry (Weinheim an der Bergstrasse, Germany) vol. 14,2 (2008): 706-13. doi:10.1002/chem.200701037
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringLipoic acid may help manage diabetes symptoms, reduce nerve pain, and support overall health.....
May 9,2025Organic ChemistryTiglic acid
80-59-1You may like
- Tiglic acid
-

- $0.00 / 25kg
- 2025-12-01
- CAS:80-59-1
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 10000KGS
- Tiglic acid
-
- $36.00 / 1kg
- 2025-09-25
- CAS:80-59-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- Tiglic acid
-

- $80.00 / 1kg
- 2025-06-03
- CAS:80-59-1
- Min. Order: 10kg
- Purity: 0.99
- Supply Ability: 20tons