Toxicity and biomonitoring of 1-ethyl-2-pyrrolidone
Sep 16,2025
Introduction
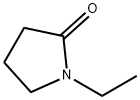
1-Ethyl-2-pyrrolidone (Figure.1; CAS 2687-91-4) is an amphiphilic, aprotic solvent used in a variety of industrial and technical applications. 1-Ethyl-2-pyrrolidone is classified as a reproductive toxicant in the European Union (EC 2013).Human metabolism of 1-ethyl-2-pyrrolidone has been previously investigated and renal conversion factors of the major urinary metabolites, 5-hydroxy-N-ethyl-2-pyrrolidone (5-HNEP) and 2-hydroxy-N-ethylsuccinimide (2-HESI), were reported. Based on these metabolites, toxicologically based biological guidance values have recently been derived (UBA 2015) and human biomonitoring of NEP exposures has been performed in occupational and environmental settings. In contrast, basic toxicokinetic data in rats and, most importantly, data on the placental transfer of 1-ethyl-2-pyrrolidone and its metabolites are missing. For N-methyl-2-pyrrolidone (NMP), as tructurally related N-alkyl-pyrrolidone, such toxicokinetic data in rats have previously been reported including NMP and metabolite levels in plasma.[1]

The toxicokinetics of 1-ethyl-2-pyrrolidone
The toxicokinetics of 1-ethyl-2-pyrrolidone (NEP), an embryotoxic organic solvent, has been studied in Sprague-Dawley rats after oral exposure. 1-Ethyl-2-pyrrolidone and its metabolites 5-hydroxy-N-ethyl-2-pyrrolidone (5-HNEP) and 2-hydroxy-N-ethylsuccinimide (2-HESI) were measured in plasma of pregnant and non-pregnant rats, and fetuses after 1-ethyl-2-pyrrolidone administration by gavage for 14 consecutive days at 50 mg/kg/day, and in plasma of non-pregnant rats after a single 1-ethyl-2-pyrrolidone administration. Additionally, amniotic fluid and 24-h urine samples of the pregnant rats were analyzed for 1-ethyl-2-pyrrolidone metabolites. Furthermore, 24-h urine samples from a repeated dose 28-day oral toxicity study in female (non-pregnant) and male rats administered developmentally non-toxic (0, 5, and 50 mg/kg/day) or toxic (250 mg/kg/day) doses of NEP were analyzed. Median peak plasma concentrations in non-pregnant rats after a single dose and repeated doses were 551 and 611 (1-ethyl-2-pyrrolidone), 182 and 158 (5-HNEP), and 63.8 and 108 µmol/L (2-HESI), respectively; whereas in pregnant rats and fetuses 653 and 619 (NEP), 80.5 and 91.7 (5-HNEP) and 77.3 and 45.7 µmol/L (2-HESI) were detected. Times to reach maximum plasma concentrations for 1-ethyl-2-pyrrolidone, 5-HNEP, and 2-HESI were 1, 4, and 8 h, respectively, and were comparable to N-methyl-2-pyrrolidone (NMP) and its corresponding metabolites. In pregnant rats, plasma elimination of 1-ethyl-2-pyrrolidone and metabolite formation/elimination was much slower compared to non-pregnant rats and efficient placental transfer of NEP was observed. Results, overall, suggest differences in the toxicokinetics of chemicals between pregnant and non-pregnant rats which need to be addressed in risk assessment, specifically when assessing developmental toxicants such as 1-ethyl-2-pyrrolidone.[1]
Toxic effects of 1-ethyl-2-pyrrolidone administered orally to rats
The developmental toxicity of 1-ethyl-2-pyrrolidone was studied in Sprague-Dawley rats after oral administration. Pregnant rats were given 1-ethyl-2-pyrrolidone at doses of 0 (distilled water), 50, 250, 500 and 750 mg/kg/day, by gavage (5 ml/kg), on gestational days (GD) 6-20. Maternal toxicity, as evidenced by reduction in body weight gain and food consumption, was observed in all NEP groups at the beginning of treatment (GD 6-9). The incidence of resorptions was significantly increased at 500mg/kg/day, and reached 83% at 750mg/kg/day. There was a dose-related decrease in fetal weight, which was significantly lower than control at 250 mg kg(-1) day(-1) and higher doses. The incidence of malformed fetuses per litter and the number of litters with malformed fetuses were significantly increased at 500 and 750mg/kg/day. Malformations mainly consisted of edema, anal atresia with absent tail, cardiovascular defects and fused cervical arches. Ossification of skull bones and sternebrae was significantly reduced at 500 and 750 mg/kg/day. The incidence of supernumerary ribs was significantly elevated at 250mg/kg/day and higher doses. In conclusion, NEP administered by gavage is embryotoxic and teratogenic at maternal toxic doses.[2]
Biomonitoring of 1-ethyl-2-pyrrolidone in automobile varnishers
N-alkyl-2-pyrrolidones are important organic solvents for varnishes in industry. This study investigates exposure to 1-ethyl-2-pyrrolidone (NEP) in varnishing of hard plastic components in an automobile plant. Two specific biomarkers of exposure, 5-hydroxy-N-ethyl-2-pyrrolidone (5-HNEP) and 2-hydroxy-N-ethylsuccinimide (2-HESI), were analyzed in urine samples of 14 workers. For this purpose, pre-shift, post-shift and next day pre-shift urine samples were collected midweek. Twelve workers performed regular work tasks (loading, wiping and packing), whereas two workers performed special work tasks including cleaning the sprayer system with organic solvents containing N-alkyl-2-pyrrolidones. Spot urine samples of nine non-exposed persons of the same plant served as controls. Median post-shift urinary levels of workers with regular work tasks (5-HNEP: 0.15 mg/L; 2-HESI: 0.19 mg/L) were ~5-fold higher compared to the controls (0.03 mg/L each). Continuously increasing metabolite levels, from pre-shift via post-shift to pre-shift samples of the following day, were observed in particular for the two workers with the special working tasks. Maximum levels were 31.01 mg/L (5-HNEP) and 8.45 mg/L (2-HESI). No clear trend was evident for workers with regular working tasks. In summary, we were able to show that workers can be exposed to 1-ethyl-2-pyrrolidone during varnishing tasks in the automobile industry.[3]
References
1.Bury D, Saillenfait AM, Marquet F, K?fferlein HU, Brüning T, Koch HM. Toxicokinetics of N-ethyl-2-pyrrolidone and its metabolites in blood, urine and amniotic fluid of rats after oral administration. Arch Toxicol. 2019;93(4):921-929. doi:10.1007/s00204-019-02404-x
2.Saillenfait AM, Gallissot F, Sabaté JP. Developmental toxic effects of N-ethyl-2-pyrrolidone administered orally to rats. J Appl Toxicol. 2007;27(5):491-497. doi:10.1002/jat.1237
3.Koslitz S, Meier S, Schindler BK, et al. Biomonitoring of N-ethyl-2-pyrrolidone in automobile varnishers. Toxicol Lett. 2014;231(2):142-146. doi:10.1016/j.toxlet.2014.10.016
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering1-Tetradecanol is a readily available, nontoxic fatty alcohol with high thermal energy storage and low under cooling. Its application researches were introduced....
Sep 16,2025Chemical Reagents1-Ethyl-2-pyrrolidone
2687-91-4You may like
1-Ethyl-2-pyrrolidone manufacturers
- NEP
-

- $800.00 / 1KG
- 2025-12-04
- CAS:2687-91-4
- Min. Order: 1G
- Purity: 99.8%
- Supply Ability: 500KG
- N-Ethyl-2-pyrrolidone
-

- $800.00 / 1KG
- 2025-12-04
- CAS:
- Min. Order: 1G
- Purity: 99.8%
- Supply Ability: 500KG
- ethyl-pyrrolidinone
-

- $800.00 / 1KG
- 2025-12-04
- CAS:
- Min. Order: 1G
- Purity: 99.8%
- Supply Ability: 500KG