What is Vancomycin?
Mar 10,2022
DESCRIPTION
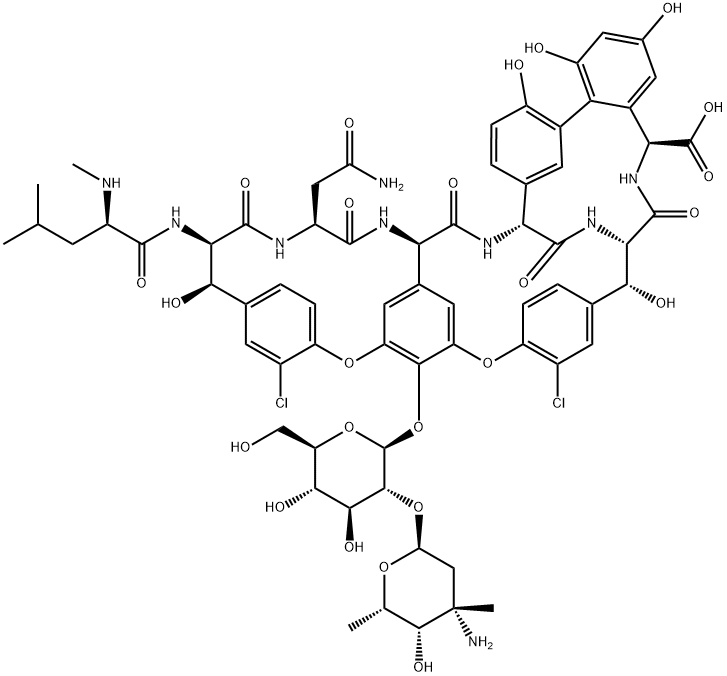
Vancomycin was isolated in the Lilly Research Laboratories from Amycolatopsis orientalis (previously designated Streptomyces orientalis and Nocardia orientalis), an organism which was found in soil in Borneo. Originally nicknamed ‘‘Mississipi mud’’ because of impurities causing a brownish color, the current vancomycin hydrochloride (Vancocins) is of more than 92% purity.
Based on its carbohydrate and peptide content, vancomycin is classified as a glycopeptide antibiotic, and it was the first member of this new class. Vancomycin is a heptapeptide, its basic structure a peptide skeleton of seven amino acids. The five amino acids at positions 2, 4, 5, 6, and 7 are aromatic (phenyl nucleus) and are more or less constant for all glycopeptides. Glycopeptides differ in the nature of amino acids in positions 1 and 3; and in the number, situation, and nature of the sugar residues (monosaccharides and disaccharides) substituted on the heptapeptide skeleton and the existence or otherwise of an acyl residue (fatty acid), such as for teicoplanin. Vancomycin belongs to group I glycopeptides according to the classification by Lancini. Like all glycopeptide antibiotics, vancomycin has a high molecular mass (1450). Its chemical formula is C66H75Cl2N9O24 HCl.
Vancomycin hydrochloride is available as a white odorless powder for dissolution and intravenous administration. The parenteral form can be administered orally for the treatment of Clostridium difficile colitis. It is also available in capsules for oral administration. Other infections have to be treated parenterally. The antibacterial spectrum includes aerobic and anerobic Gram-positive bacteria. Glycopeptides inhibit bacterial cell wall peptoglycan synthesis.
MECHANISM OF DRUG ACTION
Vancomycin is bactericidal for most Gram-positive organisms, except enterococci. Resistance of Gram-negative bacilli to vancomycin is due to a permeability barrier provided by porin proteins in the outer membrane to its large mass.
Vancomycin is bound rapidly and irreversibly to the cell walls of susceptible bacteria, thereby inhibiting cell wall synthesis. Vancomycin has a three-dimensional pocket shape which is the result of formation of bonds between its aromatic amino acid residues. The N-terminal amino acid leucine is critical for the activity of vancomycin. Vancomycin binds by its N-terminal end to the C-terminal D-Ala-D-Ala residues of the peptidoglycan precursor UDP-N-acetylmuramyl pentapeptide at the external surface of the cytoplasmic membrane. In the absence of vancomycin, the peptidoglycan precursor is added to the growing peptidoglycan chain by a transglycosylase enzyme, but vancomycin inhibits this reaction, probably due to steric hindrance. Vancomycin also inhibits the transpeptidases and carboxypeptidases that ordinarily cross-link adjacent peptidoglycan chains with pentaglycine side chains. The end result is inhibition of synthesis of the normally rigid cell wall. Lysis of the cell eventually occurs due to the unopposed action of autolysins. Inhibition of cell wall synthesis by vancomycin precedes and is different from the actions of beta-lactam agents on the cell wall. In addition, vancomycin has other actions on the permeability of the cytoplasmic membrane of S. aureus and inhibition of RNA synthesis has been ascribed to vancomycin.
Drug interactions
Co-administration of vancomycin with anesthetic agents may be associated with erythema and histamine-like flushing and anaphylactoid reactions. Concurrent and/or sequential use of potentially neurotoxic and/or nephrotoxic drugs, such as amphotericin B, aminoglycosides, bacitracin, polymyxin B, colistin, viomycin, or cisplatin, requires careful monitoring. Experience in one patient who had a persistent S. aureus bacteremia while receiving vancomycin suggested that heparin can inactivate vancomycin if the two agents are administered in a single i.v. line. Tests of the drugs in vitro at the concentrations achieved in the i.v. line resulted in precipitate formation and 50–60% reduction in vancomycin activity.
- Related articles
- Related Qustion
Iclaprim (previously known as AR-100 and Ro 48-2622) is a new member of the diaminopyrimidine class of antimicrobials, to which trimethoprim also belongs.....
Mar 10,2022InhibitorsThis nitrofuran has in vitro activity against S. aureus, S. pyogenes, Enterococcus faecalis, E. coli, Vibrio cholerae, Clostrida spp., Klebsiella, Enterobacter, Salmonella, Shigella, Campylobacter, and Bacteroides spp. However, the Vibrio c....
Mar 10,2022APIVancomycin
1404-90-6You may like
- Vancomycin
-

- 2026-02-27
- CAS:1404-90-6
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Vancomycin
-

- $42.00 / 25mg
- 2026-02-26
- CAS:1404-90-6
- Min. Order:
- Purity: 99.52%
- Supply Ability: 10g
- Vancomycin
-

- $0.00 / 1kg
- 2026-02-25
- CAS:1404-90-6
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 1000kg