|
H&D Impurities Sales Manager: Manager Sun
H&D Impurity consultation phone: +8613627253706 (wechat same number)
H&D Impurity enterprise QQ: 2881497699
The company focuses on providing pharmaceutical research and development units with new drug and generic drug registration and application of chemical products, including drug standards, drug impurity reference products, chemical reagents, the one-time import of original reference preparations, joint laboratory development of drugs and intermediates process development and production, compound custom synthesis services, as well as drug impurity preparation separation and purification, unknown impurity preparation separation and structure Appraise.
Can provide COA quality report, hydrogen spectrum, mass spectrometry, liquid phase (HPLC), can also provide carbon spectrum, ultraviolet, infrared, two-dimensional spectrum.
The default delivery standard of our MOLCOO products is: the purity of liquid phase (HPLC) is not less than 95...
H&D Impurities Sales Manager: Manager Sun
H&D Impurity consultation phone: +8613627253706 (wechat same number)
H&D Impurity enterprise QQ: 2881497699
The company focuses on providing pharmaceutical research and development units with new drug and generic drug registration and application of chemical products, including drug standards, drug impurity reference products, chemical reagents, the one-time import of original reference preparations, joint laboratory development of drugs and intermediates process development and production, compound custom synthesis services, as well as drug impurity preparation separation and purification, unknown impurity preparation separation and structure Appraise.
Can provide COA quality report, hydrogen spectrum, mass spectrometry, liquid phase (HPLC), can also provide carbon spectrum, ultraviolet, infrared, two-dimensional spectrum.
The default delivery standard of our MOLCOO products is: the purity of liquid phase (HPLC) is not less than 95%, and if the impurity is less than 95%, which is very difficult to purify, we will tell the customer specially when quoting. The project has a complete set of impurities, off-the-shelf supply,1-2 weeks of goods.
Add my contact information and provide the impurity chemical structure formula and CAS number of many items. These impurity structural formulas are very helpful to your R&D personnel. The R&D personnel can see the popular impurity structural formulas of many projects on our website, which can reduce the time for your R&D personnel to speculate the chemical structural formulas.
New and old customers we can also provide spectrum analysis services, provide synthetic routes. Off the shelf, 1~2 weeks of goods.
|
|
|
Business Scope: The company focuses on providing pharmaceutical research and development units with new drug and generic drug registration and application of chemical products, including drug standards, drug impurity reference products, chemical reagents, the one-time import of original reference preparations, joint laboratory development of drugs and intermediates process development and production, compound custom synthesis services, as well as drug impurity preparation separation and purification. Type of Enterprise: new high-tech enterprise Location: United Kingdom Germany India Founded Date: 2014-09-01 |
|
| Country: | China |
|---|---|
| Tel: | |
| Mobile: | 13627253706 |
| E-mail: | sale@hdimpurity.com |
| QQ: | 28814976 |
| Skype: | Chat Now! |
| Address: | Biwangxin Technology Building 503, Biwangxin High-tech Industrial Park, Xili Street, Nanshan District, Shenzhen |
-
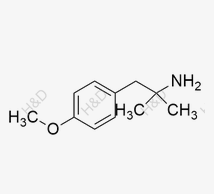
Olodaterol Impurity 11
CAS:56490-94-9 -
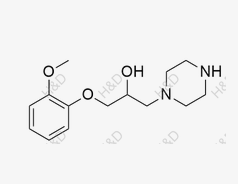
Ranolazine Impurity 6
CAS:162712-35-8 -
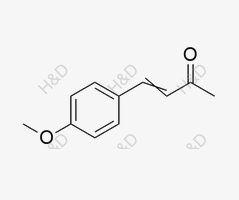
Dobutamine Impurity 25
CAS:943-88-4 -
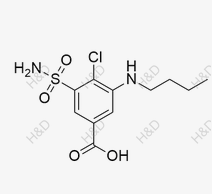
Bumetanide Impurity 31
CAS:22893-29-4 -
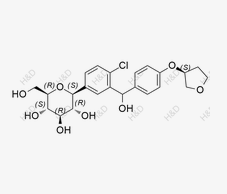
Empagliflozin Impurity YHQ
CAS:2137418-13-2 -
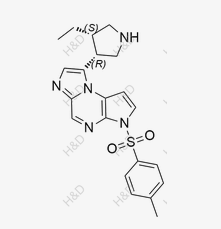
Miglitol Isomer B
CAS:132310-34-0 -
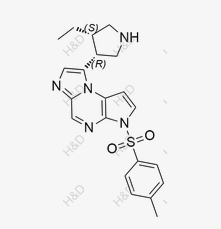
Upadacitinib Impurity 20
CAS:1428243-28-0 -
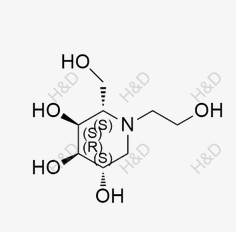
Miglitol Isomer C
CAS:1550201-56-3









