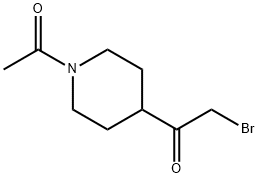
1-(1-acetyl-4-piperidinyl)-2-bromoEthanone synthesis
- Product Name:1-(1-acetyl-4-piperidinyl)-2-bromoEthanone
- CAS Number:162368-02-7
- Molecular formula:C9H14BrNO2
- Molecular Weight:248.12
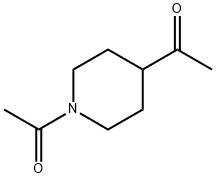
162368-01-6
49 suppliers
$45.00/50mg

162368-02-7
3 suppliers
inquiry
Yield:162368-02-7 80.23%
Reaction Conditions:
Stage #1: N-acetyl-1-(piperidin-4-yl)ethanonewith bromine in methanol; for 3 h;
Stage #2: with water;sodium carbonate in ethyl acetate;
Steps:
1.C
Step C: Preparation of l-(l-acetylpiperidin-4-yl)-2-bromoethanone: 1,1'-(Piperidine-l,4-diyl)diethanone (38.00 g, 224.6 mmol) was dissolved in methanol (700 mL) and bromine (12.11 mL, 235.8 mmol) was added in portions. The resulting mixture was agitated 3 hours and the solvent was removed in vacuo. The resulting solid was triturated with ethyl acetate and partitioned between ethyl acetate and sodium carbonate solution. The organic phase was separated, washed with brine, dried and evaporated to give the desired product (44.70 g, 80.23% yield) as yellow solid.
References:
WO2008/91770,2008,A1 Location in patent:Page/Page column 31
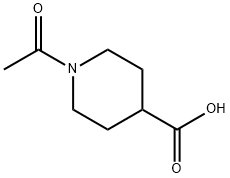
25503-90-6
301 suppliers
$5.00/1g

162368-02-7
3 suppliers
inquiry

213886-98-7
7 suppliers
inquiry

162368-02-7
3 suppliers
inquiry
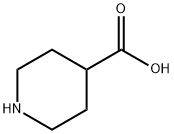
498-94-2
494 suppliers
$5.00/10g

162368-02-7
3 suppliers
inquiry