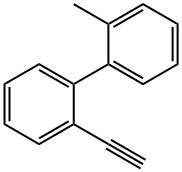
1,1'-Biphenyl, 2-ethynyl-2'-methyl- synthesis
- Product Name:1,1'-Biphenyl, 2-ethynyl-2'-methyl-
- CAS Number:1449574-70-2
- Molecular formula:C15H12
- Molecular Weight:192.26
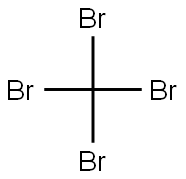
558-13-4
256 suppliers
$10.00/1g
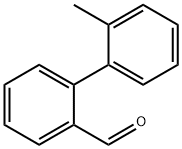
7111-68-4
41 suppliers
$53.20/250mg

1449574-70-2
2 suppliers
inquiry
Yield:1449574-70-2 1.02 g
Reaction Conditions:
Stage #1: carbon tetrabromidewith triphenylphosphine in dichloromethane at 0;Inert atmosphere;Corey-Fuchs Alkyne Synthesis;
Stage #2: 2'-methylbiphenyl-2-carboxaldehyde in dichloromethane at 0;Inert atmosphere;Corey-Fuchs Alkyne Synthesis;
Stage #3: with n-butyllithium in tetrahydrofuran;hexane at -78;Inert atmosphere;Corey-Fuchs Alkyne Synthesis;
Steps:
4.4 General procedure C for the synthesis of alkynes 3 [25]
General procedure: A 2.5 M n-BuLi solution of in hexane (2.5 equiv.) was added to a solution of 2’-(2,2-Dibromovinyl)biphenyl derivative, 2a-d (1.0 g, 1 equiv.) in THF (15 mL) at -78 °C. Stirring was continued at that temperature for 5 h under N2 atmosphere. The cold mixture was quenched with water and the aqueous phase was extracted with diethyl ether (3 × 10 mL). The combined organic layers were dried over Na2SO4 and concentrated in vacuo before the crude product was purified by flash chromatography (heptane/ethyl acetate 98:2) to give the alkyne 3a-d.
References:
Tiruye, Hiwot M.;J?rgensen, K?re B. [Tetrahedron,2022,vol. 129,art. no. 133144]

7111-68-4
41 suppliers
$53.20/250mg
90965-06-3
208 suppliers
$30.25/1g

1449574-70-2
2 suppliers
inquiry
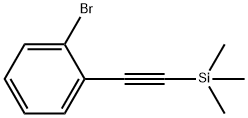
38274-16-7
81 suppliers
$28.03/1g

1449574-70-2
2 suppliers
inquiry
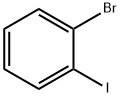
583-55-1
484 suppliers
$7.00/5g

1449574-70-2
2 suppliers
inquiry
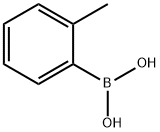
16419-60-6
378 suppliers
$9.00/1g

1449574-70-2
2 suppliers
inquiry