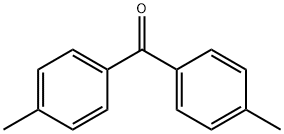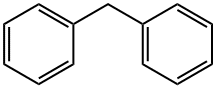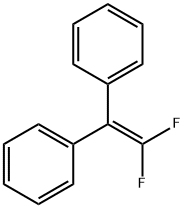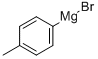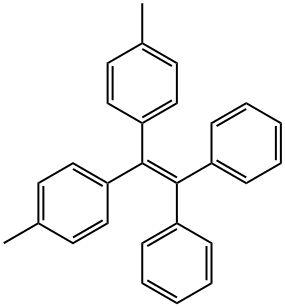
1,1-diphenyl-2,2-di(p-tolyl)ethylene synthesis
- Product Name:1,1-diphenyl-2,2-di(p-tolyl)ethylene
- CAS Number:32298-40-1
- Molecular formula:C28H24
- Molecular Weight:360.49
Yield:32298-40-1 85%
Reaction Conditions:
Stage #1: Diphenylmethanewith n-butyllithium in tetrahydrofuran at 0; for 0.5 h;Inert atmosphere;
Stage #2: bis(p-methylphenyl)-methanonewith n-butyllithium in tetrahydrofuran at 20; for 6 h;Inert atmosphere;
Stage #3: with toluene-4-sulfonic acid in toluene at 90; for 5 h;Inert atmosphere;
Steps:
1 Example 1: Synthesis of Compound 3
At 0°C under N2 atmosphere, 37.1 mL of n-butyllithium (1.6M) was added to a solution of compound 1 (10 g, 59.4 mmol) in THF (100 mL), and the resulting orange-red solution was stirred at 0°C for 30 min. Then, compound 2 (6.3 g, 29.7 mmol) was dissolved in THF (20 mL) to obtain a THF solution of compound 2, and then the solution of compound 2 was added dropwise to the mixed solution of compound 1 and n-butyllithium, and the mixed solution was heated up To room temperature, stirring was continued for 6 h. The reaction was quenched with 60 mL of saturated aqueous ammonium chloride.Extraction with DCM The organic phase was dried over anhydrous Na2SO4.The obtained crude product was dissolved in 20 mL of toluene, a catalytic amount of p-toluenesulfonic acid (342 mg, 1.8 mmol) was added, the reaction was refluxed at 90° C. for 5 h, and the generated water was separated with a molecular sieve dehydration device. The toluene mixed solution was washed with a 10% NaHCO3 aqueous solution by mass, dried with anhydrous Na2SO4, and then removed by rotary evaporation. The obtained crude product was purified by column chromatography (silica gel; It was deliquored, spin-dried, and then vacuum-dried at 50° C. for 12 h to obtain a white solid 3 (9.0 g, yield 85%).
References:
CN114177175,2022,A Location in patent:Paragraph 0020; 0041-0042
2592-73-6
6 suppliers
inquiry

32298-40-1
7 suppliers
inquiry

859777-49-4
0 suppliers
inquiry

32298-40-1
7 suppliers
inquiry