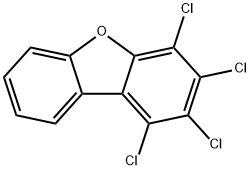
1,2,3,4-TETRACHLORODIBENZOFURAN synthesis
- Product Name:1,2,3,4-TETRACHLORODIBENZOFURAN
- CAS Number:24478-72-6
- Molecular formula:C12H4Cl4O
- Molecular Weight:305.97
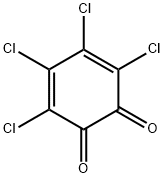
2435-53-2
150 suppliers
$86.90/5g
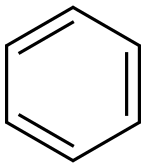
71-43-2
718 suppliers
$11.19/25ML

24478-72-6
9 suppliers
$501.00/5MG
Yield:24478-72-6 41%
Reaction Conditions:
with trifluorormethanesulfonic acid in 2,2,2-trifluoroethanol at -30 - 80; for 18 h;Inert atmosphere;Solvent;Temperature;
Steps:
Procedure for the Synthesis of Dibenzofurans
General procedure: Under an inert atmosphere, a solution is prepared containing triflic acid (1 mL, 11 mmol), arene (1 g, ca. 8 mmol), and co-solvent (1 mL of CH2Cl2 or CF3CH2OH) o-Chloranil (0.15 g, 0.6 mmol). The mixture is cooled to -30 °C and a solution of o-chloranil 5 (in 2 mL co-solvent) is added slowly with stirring. The resulting mixture is allowed to slowly warm to room temperature overnight. Following this reaction period, the mixtureis poured over ice and the solution is extracted three times with chloroform. The organic extracts are then washed twice with water and twice with brine solution. Following a drying regiment with anhydrous Na2SO4, the organic solvent is removed and the crude product is subject to chromatography with hexanes.
References:
Qarah, Ahmad;Gasonoo, Makafui;Do, Dat;Klumpp, Douglas A. [Tetrahedron Letters,2016,vol. 57,# 33,p. 3711 - 3714] Location in patent:supporting information
190-26-1
18 suppliers
inquiry

24478-72-6
9 suppliers
$501.00/5MG
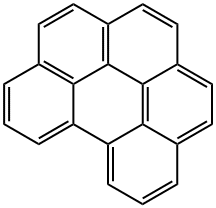
191-24-2
238 suppliers
$24.00/100mg

24478-72-6
9 suppliers
$501.00/5MG
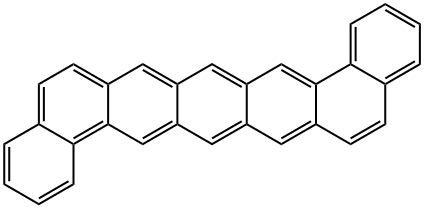
227-09-8
41 suppliers
$40.00/10mg

24478-72-6
9 suppliers
$501.00/5MG
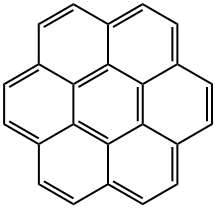
191-07-1
237 suppliers
$39.00/100mg

24478-72-6
9 suppliers
$501.00/5MG