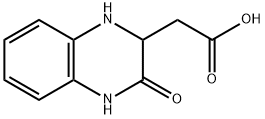
1,2,3,4-TETRAHYDRO-3-OXO-2-QUINOXALINEACETIC ACID synthesis
- Product Name:1,2,3,4-TETRAHYDRO-3-OXO-2-QUINOXALINEACETIC ACID
- CAS Number:136584-14-0
- Molecular formula:C10H10N2O3
- Molecular Weight:206.2
56-84-8
938 suppliers
$5.00/25g
1493-27-2
510 suppliers
$5.00/10g

136584-14-0
64 suppliers
$45.00/50mg
Yield:-
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;aluminum nickel in methanol;2-methoxy-ethanol;water
Steps:
XXVIII 3-Carboxymethyl-3,4-dihydroquinoxalin-2(1H)-one
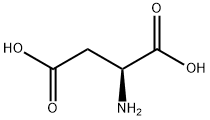
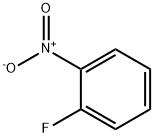
EXAMPLE XXVIII 3-Carboxymethyl-3,4-dihydroquinoxalin-2(1H)-one 2-Fluoronitrobenzene (14.1 g, 0.1 mol) and L-aspartic acid (40.0 g, 0.3 mol) were heated to 95° C. in 100 ml of 2-methoxyethanol, with stirring, and 300 ml of 2N sodium hydroxide solution were added dropwise. Stirring was then continued for 1 hour at this temperature. After the solution had cooled, it was treated with 500 ml of methanol and hydrogenated under atmospheric pressure with Raney nickel as catalyst. When the uptake of hydrogen had ended, the catalyst was removed by filtration with suction, and the solution was concentrated under reduced pressure. The residue was acidified with 500 ml of 2N hydrochloric acid, the mixture was subsequently concentrated, neutralized with sodium acetate and extracted with ethyl acetate. The mixture was dried with sodium sulfate, the solvent was stripped off, and the residue was then obtained which was first oily and crystallized upon stirring with water, melting point 152-154° C. 1H NMR (60 MHz, d6-DMSO): δ=2.5-2.7 (dd partly concealed, 2 H), 4.1 (td, J=6, 2 Hz, 1 H), 5.98 (br. s, 1 H), 6.5-6.9 (m, 4 H), 10.30 (br. s, 1 H), 12.37 ppm (br. s, 1 H). MS: M+=206; CHN analysis: calculated C, 58.2; H, 4.8; N 13.6%; found C, 58.4; H, 4.7; N, 13.7%.
References:
Aventis Pharma Deutschland GmbH US6369057, 2002, B1
136584-15-1
3 suppliers
inquiry

136584-14-0
64 suppliers
$45.00/50mg