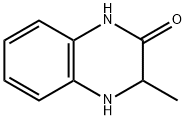
3-METHYL-3,4-DIHYDRO-2(1H)-QUINOXALINONE synthesis
- Product Name:3-METHYL-3,4-DIHYDRO-2(1H)-QUINOXALINONE
- CAS Number:34070-68-3
- Molecular formula:C9H10N2O
- Molecular Weight:162.19
Yield:34070-68-3 330 mg
Reaction Conditions:
with triethylamine in tetrahydrofuran at 65;
Steps:
3-(2-Methyl-3-oxo-1,2,3,4-tetrahydroquinoxaline-1-carbonyl)benzoic Acid (6): Beige Solid
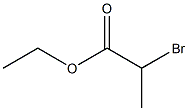
General procedure: This compound was prepared in 3 steps from o-phenylenediamine.(a) To a solution of o-phenylenediamine (200 mg, 1.85 mmol) in dry THF (3.7 ml) is added triethylamine (260 l, 1.85 mmol) followed by D,L-ethyl-2-bromopropionate (240 l, 1.85 mmol). The reaction is heated to 65°C overnight. The reaction is quenched with water and extracted with ethyl acetate (3x). The combined organic layers are dried over magnesium sulfate, filtered and the solvent removed under reduced pressure. 3-Methyl-3,4-dihydroquinoxalin-2(1H)-one (5) (330 mg) is obtained in quantitative yield in 93% purity. (b) To a solution of 3-methyl-3,4-dihydroquinoxalin-2(1H)-one (5) (200 mg, 1.23mmol) in dry THF (1 ml) is added triethylamine (189 l,1.35 mmol) followed by a solution of methyl 3-(chlorocarbonyl)benzoate (282 mg, 1.41 mmol) in dry THF(1.4 ml). The reaction is stirred at room temperature for 4 hours. The reaction is diluted with ethyl acetate, washed with 1 M HCl and 1 M NaHCO3. The organic layer is dried over magnesium sulfate, filtered and the solvent removed under reduced pressure. The crude product is purified via silicagel chromatography: dichloromethane/methanol 98/2. Methyl3-(2-methyl-3-oxo-1,2,3,4-tetrahydroquinoxaline-1-carbonyl)benzoate (384 mg) is obtained in 96% yield. (c) To a solution of methyl 3-(2-methyl-3-oxo-1,2,3,4-tetrahydroquinoxaline-1-carbonyl)benzoate (200 mg, 0.62 mmol) in dry THF (6 ml) is added potassium trimethylsilanolate (396mg 3.08 mmol). The reaction is stirred under argon. The reaction is diluted with ethyl acetate and washed with water,the aqueous layer is acidified with 1M HCl and extracted with ethyl acetate. The organic layer is dried over magnesium sulfate, filtered and the solvent removed under reduced pressure. The crude product is purified via silicagel chromatography: dichloromethane/methanol 96/4. 3-(2-Methyl-3-oxo-1,2,3,4-tetrahydroquinoxaline-1-carbonyl)benzoic acid (65 mg) is obtained in 99% purity and 34% yield as a beige solid. 1H NMR (DMSO-d6, 500 MHz) 10.84 (s, 1H,COOH), 7.99 (d, J = 7.7 Hz, 1H, H-1), 7.89 (s, 1H, H-16),7.53 (m, 1H, H-16), 7.46 (t, 1H, J = 7.6 Hz, H-2), 7.07 (t, J= 7.1 Hz, 1H, H-15), 7.02 (d, J = 6.6 Hz, 1H, H-14), 6.67 (m,2H, H-3, H-4), 4.95 (bs, 1H, H-10), 1.19 (d, J = 7,2 Hz 3H,CH3); LC-MS ESI (pos): m/z 311 [M + H]+.
References:
Tsimafeyeu, Ilya;Daeyaert, Frits;Joos, Jean-Baptiste;Aken, Koen V.;Ludes-Meyers, John;Byakhov, Mikhail;Tjulandin, Sergei [Medicinal Chemistry,2016,vol. 12,# 4,p. 303 - 317]
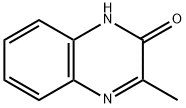
14003-34-0
84 suppliers
$53.00/250mg

34070-68-3
63 suppliers
$28.60/10MG
600-22-6
392 suppliers
$6.00/5g
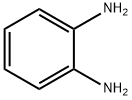
95-54-5
541 suppliers
$9.00/1g

14003-34-0
84 suppliers
$53.00/250mg

34070-68-3
63 suppliers
$28.60/10MG
![Spiro[oxazole-4(5H),2'(3'H)-quinoxalin]-3'-one, 1',4'-dihydro-2-phenyl-](/CAS/20210305/GIF/215191-49-4.gif)