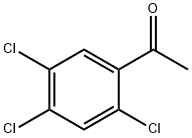
1-(2,4,5-trichlorophenyl)ethan-1-one synthesis
- Product Name:1-(2,4,5-trichlorophenyl)ethan-1-one
- CAS Number:13061-28-4
- Molecular formula:C8H5Cl3O
- Molecular Weight:223.48

75-36-5
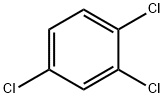
120-82-1

13061-28-4
Acetyl chloride (11.8 g, 150 mmol) was slowly added dropwise to a stirred mixture of 1,2,4-trichlorobenzene (18.2 g, 100 mmol) and anhydrous aluminum trichloride (33.4 g, 250 mmol) at room temperature. The reaction mixture was then gradually heated to 120-125°C (oil bath heating) and the reaction was maintained at this temperature for 3 hours. Upon completion of the reaction, the resulting viscous mixture was carefully poured into ice water (125 mL) to quench the reaction, followed by extraction of the aqueous phase with dichloromethane (3 x 70 mL). The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and the solvent concentrated under reduced pressure. The residue was purified by distillation under reduced pressure to give a colorless liquid product which solidified to a solid after standing at room temperature. Yield: 15.6 g (82% yield); melting point: 43-45 °C, boiling point: 135 °C/6 mmHg (literature values: melting point 47 °C [29]; 44-46 °C [30]; 45.5-46 °C [31], boiling point 114-116 °C/2 mmHg [31]).

75-36-5
589 suppliers
$17.92/100G

120-82-1
322 suppliers
$10.00/25g

13061-28-4
50 suppliers
$48.00/100mg
Yield: 82%
Reaction Conditions:
with aluminum (III) chloride at 20; for 3 h;
Steps:
4.1 2,4,5-Trichloroacetophenone (4)
To a stirred mixture of 1,2,4-trichlorobenzene (18.2 g,100mmol) and anhydrous aluminum trichloride (33.4 g,250mmol), acetyl chloride (11.8 g, 150mmol) was dropwiseadded at room temperature. The reaction mixturewas gradually heated to 120-125°C (oil bath) and maintainedat this temperature for 3h. The resulting viscousmixture was cautiously poured onto ice-water (125mL), and the aqueous mixture was extracted with dichloromethane(3×70 mL). The combined organic extractswere dried (MgSO4), evaporated, and the residue wasdistilled under reduced pressure to give a colorless liquidwhich solidified upon standing at room temperature.Yield: 15.6 g (82%); m. p. 43-45°C, b. p. 135°C/6mmHg (lit.[29]: m.p. 47°C; lit. [30]: m. p. 44-46°C; lit. [31]: 45.5-46°C,b. p. 114-116°C/2mmHg).
References:
Barhoumi, Lina M.;El-Abadelah, Mustafa M.;Sabri, Salim S.;Voelter, Wolfgang [Zeitschrift fur Naturforschung, B: Chemical Sciences,2017,vol. 72,# 5,p. 369 - 375]
50-82-8
111 suppliers
$14.14/250mgs:

13061-28-4
50 suppliers
$48.00/100mg
6575-04-8
11 suppliers
inquiry

13061-28-4
50 suppliers
$48.00/100mg
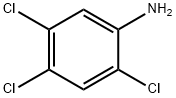
636-30-6
226 suppliers
$31.00/25g

13061-28-4
50 suppliers
$48.00/100mg
18815-73-1
1 suppliers
inquiry
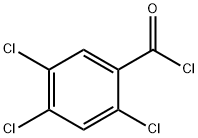
42221-49-8
14 suppliers
$95.00/250mg

13061-28-4
50 suppliers
$48.00/100mg