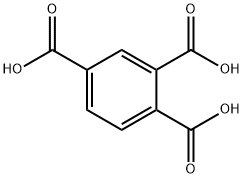
1,2,4-Benzenetricarboxylic acid synthesis
- Product Name:1,2,4-Benzenetricarboxylic acid
- CAS Number:528-44-9
- Molecular formula:C9H6O6
- Molecular Weight:210.14
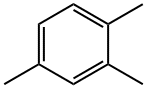
95-63-6
293 suppliers
$14.80/250mg
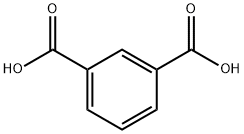
121-91-5
607 suppliers
$6.00/10g
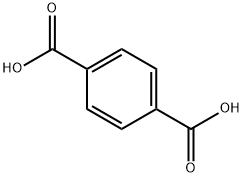
100-21-0
591 suppliers
$6.00/25g

528-44-9
226 suppliers
$11.00/5g
Yield:528-44-9 88.3%
Reaction Conditions:
with oxygen;titanium(IV) isopropylate;tetrabutoxytitanium;manganese(II) acetate;cobalt(II) acetate;ammonium bromide;cerous nitrate in water;acetic acid at 150 - 225; under 5250.53 - 18751.9 Torr; for 1.2 - 1.25 h;Product distribution / selectivity;
Steps:
1; 2
EXAMPLE 1 In the autoclave were introduced 1212 g of acetic acid (water content 5.6 wt %), 4.19 g of cobalt acetate tetrahydrate, 2.82 g of manganese acetate tetrahydrate, 1.09 g of ammonium bromide, 0.77 g of cerium nitrate hexahydrate and 0.87 g of a mixture of tetraisopropyl titanate and tetrabutyl titanate (Ti content 16 wt %).The autoclave was closed and nitrogen was fed to remove air. Temperature and pressure were increased to 8 bar and 160° C. under stirring before starting pseudocumene and air feed.540 g of pseudocumene were fed in 14 minutes by a piston metering pump; air flow was regulated through a mass flow meter at 2380 NL/h.After 14 minutes the pseudocumene feed was stopped while the air feed was continued at the same rate.At this point the oxygen consumed by the reaction corresponded to 20% of the amount required for the complete oxidation of pseudocumene to trimellitic acid.After stopping the pseudocumene feed, the pressure was gradually increased up to 25 bar in order to raise the temperature up to 225° C.After about 60 minutes from the start the air flow was gradually reduced in order to keep the O2 content in the off-gases below 5.0 vol %. The total duration of the run was 75 minutes.After cooling down to room temperature and depressurizing to ambient pressure, the product of the reaction was analyzed by gas chromatography (GC) and high pressure liquid chromatography (HPLC) for the organic components.The conversion of pseudocumene was complete. The molar yields of desired product and by-products are given in Table 1.After filtration the crude TMA filter cake was dried and analyzed by HPLC. The weight composition of the dried solid is given in Table 1. It can be noted that, beside the good yield, the formation of the undesired bi-functional byproducts isophthalic and terephthalic acid is low and the purity of crude trimellitic acid is high (around 98 wt %).; EXAMPLE 2 The procedure of Example 1 was repeated but the temperature of the semi-continuous stage was 150° C. and the pressure 7 bar.At the end of the semi-continuous stage the oxygen consumed by the reaction corresponded to 19% of the amount required for the complete oxidation of pseudocumene to trimellitic acid.The yields and the product composition are given in Table 1.; COMPARATIVE EXAMPLE 1 Semi-Continuous, Staged Addition of the CatalystThe reaction was carried out as described in Example 1 but the catalyst addition was staged. Before starting the feed of pseudocumene in the reactor were introduced 1212 g of acetic acid (water content 5.6 wt %), 2.81 g of cobalt acetate tetrahydrate, 1.89 g of manganese acetate tetrahydrate, 0.732 g of ammonium bromide, 0.515 g of cerium nitrate hexahydrate and 0.583 g of a mixture of tetraisopropyl titanate and tetrabutyl titanate (Ti content 16%).The autoclave was closed and the air was displaced with nitrogen. Temperature and pressure were increased to 8 bar and 160° C. under stirring, before starting pseudocumene and air feed. 540 g of pseudocumene were fed in 14 minutes by a piston metering pump; the air flow was regulated through a mass flow meter at 2380 NL/h.At this point the oxygen consumed by the reaction corresponded to 20% of the amount required for the oxidation of pseudocumene to trimellitic acid.After completion of the pseudocumene feed the air feed was continued at the same rate and the pressure was gradually increased up to 25 bar in order to raise the temperature up to 224° C. and then an aqueous solution of cobalt acetate tetrahydrate, manganese acetate tetrahydrate, ammonium bromide and cerium nitrate hexahydrate was fed to the reactor till the end of the reaction. The total amount of salts added was: 1.53 g of cobalt acetate tetrahydrate, 1.03 g of manganese acetate tetrahydrate, 0.397 g of ammonium bromide, 0.279 g of cerium nitrate hexahydrate and 16 g of water.After about 60 minutes from the start the air flow was gradually reduced in order to keep the O2 content in the off-gases below 5.0 vol %. Total duration of the run was 72 minutes.After cooling down to room temperature and depressurizing to ambient pressure, the product of the reaction was analyzed by GC and HPLC for the organic components.The conversion of pseudocumene was complete. The yields and the product composition are given in Table 2.; COMPARATIVE EXAMPLE 2 Semi-Continuous, High Conversion at the End of the Semi-Continuous StageThe procedure of Example 1 was repeated but the temperature of the semi-continuous stage was 170° C., the pressure 9 bar and pseudocumene was fed in 20 minutes. At the end of the feed of pseudocumene, the oxygen consumed by the reaction corresponded to 30% of the amount required for the oxidation of pseudocumene to trimellitic acid.The yields and the product composition are given in Table 2.; COMPARATIVE EXAMPLE 3 Batch, Staged Addition of the CatalystThe procedure of Example 1 was repeated, but all the pseudocumene was loaded at the beginning, together with the solvent. The catalyst was added partly at the beginning and partly in the second part of the reaction. At the beginning were loaded into the reactor 4.18 g of cobalt acetate tetrahydrate, 2.64 g of manganese acetate tetrahydrate, 0.54 g of ammonium bromide, 0.118 g of cerium nitrate hexahydrate and 0.866 g of a mixture of tetraisopropyl titanate and tetrabutyl titanate (Ti content 16%).After 1 minute, an aqueous solution of manganese acetate tetrahydrate, ammonium bromide, and cerium acetate hexahydrate was fed to the reactor till the end of the reaction. Total duration of the run was 75 minutes. The total amount of salts added was: 0.166 g of manganese acetate tetrahydrate, 0.529 g of ammonium bromide, 0.627 g of cerium nitrate hexahydrate and 12 g of water.After cooling down to room temperature and depressurizing to ambient pressure, the product of the reaction was analyzed by GC and HPLC for the organic components.The yields and the product composition are given in Table 2.
References:
Castiglioni, Gian Luca;Pirola, Roberto;Fumagalli, Carlo US2009/43124, 2009, A1 Location in patent:Page/Page column 3-4
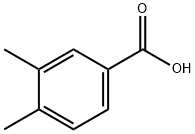
619-04-5
257 suppliers
$5.00/250mg

528-44-9
226 suppliers
$11.00/5g
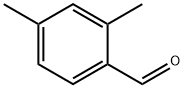
15764-16-6
207 suppliers
$7.00/1g

528-44-9
226 suppliers
$11.00/5g
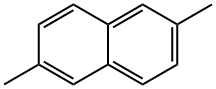
581-42-0
177 suppliers
$50.00/1 g
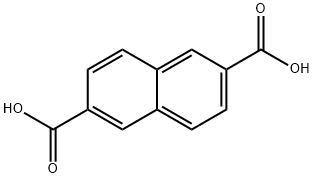
1141-38-4
258 suppliers
$5.00/250mg

528-44-9
226 suppliers
$11.00/5g
2459-10-1
71 suppliers
$10.00/1g

528-44-9
226 suppliers
$11.00/5g