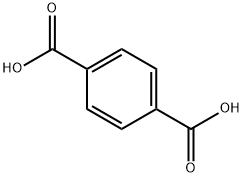
Terephthalic acid synthesis
- Product Name:Terephthalic acid
- CAS Number:100-21-0
- Molecular formula:C8H6O4
- Molecular Weight:166.13
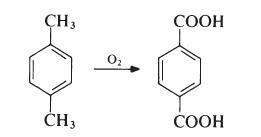
p-Xylene is obtained largely from petroleum sources, being a product of the fractionation of reformed naphthas. The oxidation is carried out in the liquid phase. Typically, air is passed into a solution of p-xylene in acetic acid at about 200℃ and 2 MPa (20 atmospheres) in the presence of a catalyst system containing cobalt and manganese salts and a source of bromide ions. The terephthalic acid produced contains only small amounts of impurities (mainly p-carboxybenzaldehyde), which are readily removed. The acid is dissolved in water at about 2500 e and 5 MPa (50 atmospheres) and treated with hydrogen (which converts the aldehyde to p-toluic acid). The solution is then cooled to 100℃ and pure terephthalic acid crystallizes.
106-42-3
434 suppliers
$19.00/25ML

100-21-0
588 suppliers
$6.00/25g
Yield:100-21-0 99.9%
Reaction Conditions:
with ammonium acetate;lithium hydroxide monohydrate;hydrogen bromide;oxygen;manganese(II) acetate;Co(OAc)2.4H2O;1-butyl-3-methyl-1H-imidazol-3-ium bromide;glacial acetic acid at 200; under 30753.1 Torr; for 10 h;Inert atmosphere;Time;Temperature;Reagent/catalyst;Concentration;
Steps:
5
[0057] Example 1 : Experimental procedure: In a fume hood, load a Parr reactor with the specified amounts of components for the given experiment seal the reactor. The Parr reactor includes a gas distributor to disperse the gas through a 1.6 mm opening into the liquid, a mechanical gas entrainment stirrer, and baffles to ensure thorough mixing. Install the Parr reactor in a heater assembly at room temperature and connect a gas supply line to the reactor and a condenser to the reactor outlet. During operation, gases exit the reactor through the condenser then a trap, then a back-pressure regulator. Connect a safety vent having a rupture disk, and thermocouples to the reactor. Connect a cooling water recirculator to the condenser and begin to recirculate cooling water. Pressure test the Parr reactor at room temperature and 1.4 MPa(g) (200 psig) using nitrogen until there is no decrease in pressure for 15 minutes. Set the back pressure regulator on the reactor outlet to the experimental pressure and pressure test the reactor under nitrogen. [0058] Begin raising the reactor temperature to the experimental temperature under the nitrogen atmosphere. Always follow all instructions for the specific reactor including temperature and pressure limits. When the reactor reaches the desired temperature begin adding air at the experimental rate and monitor the reactor temperature and pressure for the duration of the test. During the test, the air flow into the reactor is maintained at 1250 or 2500 standard cm3 per minute, the pressure is maintained at 4.1 MPa(g), and the stirrer is maintained at 1600 rpm. At the end of the test shut off the heater, cut the air flow and allow the reactor to cool. When the reactor cools to less than 35°C, open the back pressure valve, stop the cooling water, and remove and empty the reactor to obtain the solid oxidation product and mother liquor. [0059] The mother liquor and products are filtered under vacuum to separate the solids and liquid. The solids are then mixed with 100 cc deionized water at room temperature and decanted. The room temperature deionized water mixing and decanting is repeated two additional times. A fourth wash with deionized water is heated to 95°C for 30 minutes and then filtered. The solids are dried at 80°C for 8-24 hours before analyzing. [0065] Example 5: Same oxidizing conditions as Example 3. l-butyl-3- methylimidazolium acetate was not used and the amounts of acetic acid, ammonium acetate and l-butyl-3 -methylimidazolium bromide were increased. Here, both the 4-CBA and p- toluic acid are significantly reduced compared to the conventional test (Example 2). The benzoic acid level is still higher than Example 2, but lower than Example 3.
References:
WO2013/101325,2013,A1 Location in patent:Paragraph 0057-0059; 0065
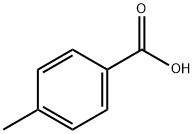
99-94-5
607 suppliers
$9.00/1g

100-21-0
588 suppliers
$6.00/25g

106-42-3
434 suppliers
$19.00/25ML

100-21-0
588 suppliers
$6.00/25g
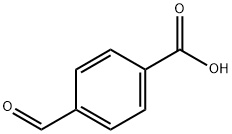
619-66-9
438 suppliers
$5.00/5g

99-94-5
607 suppliers
$9.00/1g