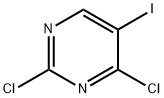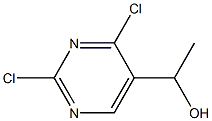
1-(2,4-dichloropyriMidin-5-yl)ethanol synthesis
- Product Name:1-(2,4-dichloropyriMidin-5-yl)ethanol
- CAS Number:130825-17-1
- Molecular formula:C6H6Cl2N2O
- Molecular Weight:193.03
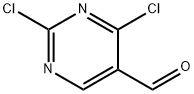
871254-61-4
155 suppliers
$21.00/100mg

75-16-1
276 suppliers
$12.00/10ml

130825-17-1
11 suppliers
inquiry
Yield:130825-17-1 97%
Reaction Conditions:
Stage #1: 2,4-dichloro-5-pyrimidinecarboxaldehyde;methylmagnesium bromide in tetrahydrofuran;diethyl ether at -78; for 2 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;diethyl ether;water;
Steps:
20 1-(2,4-Dichloro-pyrimidin-5-yl)-ethanol
Example 20 1-(2,4-Dichloro-pyrimidin-5-yl)-ethanol To a stirred solution of 2,4-dichloro-pyrimidine-5-carbaldehyde (Example 17, 1.01 g, 5.7 mmol) in THF (10 mL), methyl magnesium bromide (Aldrich, 3 M in ether, 6.27 mmol, 2.1 mL) was added slowly at -78° C. The mixture was stirred for an additional 2 hours at -78° C. and the reaction was quenched with 1N HCl (10 mL). The resulting mixture was extracted with EtOAc and the extract was dried with sodium sulfate. Removal of solvent gave a brown solid. 1.07 g, 97%. MS (M+H)+, 193.
References:
US2005/277655,2005,A1 Location in patent:Page/Page column 16
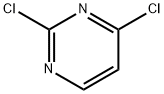
3934-20-1
725 suppliers
$9.00/1g

130825-17-1
11 suppliers
inquiry

3934-20-1
725 suppliers
$9.00/1g

75-07-0
428 suppliers
$14.00/5mL

130825-18-2
0 suppliers
inquiry

130825-17-1
11 suppliers
inquiry