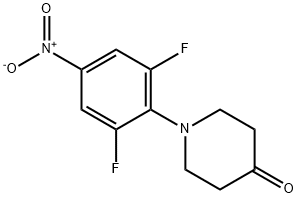
1-(2,6-difluoro-4-nitrophenyl)piperidin-4-one synthesis
- Product Name:1-(2,6-difluoro-4-nitrophenyl)piperidin-4-one
- CAS Number:565459-90-7
- Molecular formula:C11H10F2N2O3
- Molecular Weight:256.21
66684-58-0
216 suppliers
$10.00/1g
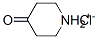
41979-39-9
0 suppliers
$15.00/10mg

565459-90-7
16 suppliers
inquiry
Yield:565459-90-7 97%
Reaction Conditions:
Stage #1: 4-piperidone hydrochloridewith triethylamine in chloroform; for 0.5 h;
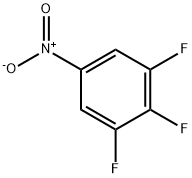
Stage #2: 3,4,5-trifluoronitrobenzene in chloroform at 65 - 70; for 8 h;
Steps:
Chloroform (9.3 L) was charged in a 20 L reaction assembly and 4-piperidone hydrochloride (1.17 Kg, 7.62 mol) was added under stirring followed by triethylamine (2.14 Kg, 2.95 L, 21.1 mol). After 30 minutes of stirring, 3,4,5-trifluoronitrobenzene (1.5 Kg, 8.47 mol) was added to the mixture in one lot and the contents were heated to 65-70°C for 8 h. After completion of the reaction, chloroform was removed under vacuum to obtain a syrupy mass. At this stage, water (10 L) was added to the mass and the chloroform recovery was continued under vacuum below 65°C till the chloroform was removed completely. The slurry was cooled to RT and filtered. The solid product was washed with water (3 L) followed by hexanes (2 L). The product was dried in a vacuum oven below 70°C to obtain the product as a yellow solid, 1.88 Kg ; Yield 97%.M.P.: 130-132°C; MS: 257(M+1); M.F.: C11H10F2N2O3.
References:
WO2012/59823,2012,A1 Location in patent:Page/Page column 10-11
41661-47-6
0 suppliers
$45.00/250mg

66684-58-0
216 suppliers
$10.00/1g

565459-90-7
16 suppliers
inquiry
![8-(2,6-DIFLUORO-4-NITROPHENYL)-1,4-DIOXA-8-AZASPIRO[4.5]DECANE](/CAS/20200331/GIF/1332356-41-8.gif)
1332356-41-8
7 suppliers
$698.00/1g

565459-90-7
16 suppliers
inquiry
![1,4-Dioxa-8-azaspiro[4.5]decane](/CAS/GIF/177-11-7.gif)
177-11-7
265 suppliers
$6.00/1g

565459-90-7
16 suppliers
inquiry

66684-58-0
216 suppliers
$10.00/1g

565459-90-7
16 suppliers
inquiry