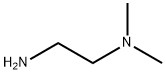
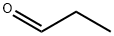1,2-Ethanediamine, N1,N1-dimethyl-N2-propyl- synthesis
- Product Name:1,2-Ethanediamine, N1,N1-dimethyl-N2-propyl-
- CAS Number:89893-80-1
- Molecular formula:C7H18N2
- Molecular Weight:130.23
Yield:89893-80-1 11%
Reaction Conditions:
Stage #1: propionaldehyde;N,N-dimethylethylenediamine in dichloromethane at 20; for 2 h;
Stage #2: with sodium tetrahydroborate in dichloromethane at 20; for 17 h;
Steps:
1.1 [Synthesis Example 1]
In a fully dried 200 mL reactor, 4 mL (36.7 mmol) of N, N-dimethylethylenediamine and 70 mL of dichloromethane were charged, and 3 mL (41.7 mmol) of propionaldehyde was added to the place where the mixture was stirred at room temperature, and at room temperature. Stirred. After 2 hours, the solvent was distilled off under reduced pressure, and the mixture was diluted with 90 mL of ethanol. Then, 1.40 g (37.1 mmol) of sodium borohydride was added, and the mixture was stirred at room temperature. After 17 hours, the solvent was distilled off under reduced pressure, and a saturated aqueous solution of ammonium chloride was added to the reaction solution to quench the reaction. The soluble component was extracted with dichloromethane, and the obtained fraction was washed with saturated brine and dried over anhydrous magnesium sulfate. After filtering magnesium sulfate, the filtrate is distilled off under reduced pressure, the crude product is distilled under reduced pressure, and the target product [hereinafter referred to as compound (B-2)] represented by the following formula (B-2) is 0. .51 g (yield 11%) was obtained
References:
JP2021/151994,2021,A Location in patent:Paragraph 0134-0135