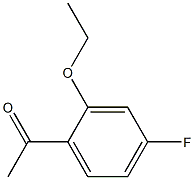
1-(2-ethoxy-4-fluorophenyl)ethanone synthesis
- Product Name:1-(2-ethoxy-4-fluorophenyl)ethanone
- CAS Number:51788-79-5
- Molecular formula:C10H11FO2
- Molecular Weight:182.1915
Yield:51788-79-5 520 kg
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 50; for 8 h;Large scale;
Steps:
3.2 Step 2. l-(2-Ethoxy-4-fluorophenyl)ethanone (iii-c)
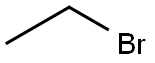
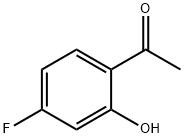
A reactor was charged with l-(4-fluoro-2-hydroxyphenyl)ethanone (ii-c, 524.8 kg), DMF (1309 kg), K2CO3 (678.3kg), and bromoethane (480.8 kg). The reaction mixture was agitated and heated at 50 °C for 8 hrs, at which time ion pair chromatography (IPC) showed NMT 1.0% of l-(4-fluoro-2-hydroxyphenyl)ethanone (ii-c). The mixture was allowed to cool to room temperature and diluted with water (3927 kg). The precipitate was collected by filtration and washed with water (800 kg). The cake was dissolved in cyclohexane (2000 kg) heated at 60 °C and the bottom aqueous layer was drained off. The solution was cooled to 15°C, aged for 1 h and filtered to give l-(2-ethoxy-4-fluorophenyl)ethanone (iii-c, 520 Kg) as a yellow solid.
References:
WO2021/158891,2021,A1 Location in patent:Page/Page column 67; 68; 69
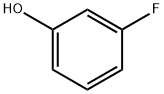
372-20-3
395 suppliers
$7.00/5g

51788-79-5
10 suppliers
inquiry