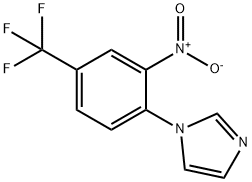
1-[2-nitro-4-(trifluoromethyl)phenyl]-1H-imidazole synthesis
- Product Name:1-[2-nitro-4-(trifluoromethyl)phenyl]-1H-imidazole
- CAS Number:25373-61-9
- Molecular formula:C10H6F3N3O2
- Molecular Weight:257.17
Yield:25373-61-9 97%
Reaction Conditions:
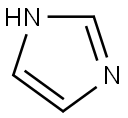
Stage #1: 1H-imidazolewith potassium tert-butylate in dimethyl sulfoxide; for 0.0833333 h;
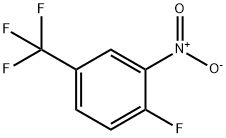
Stage #2: 1-fluoro-2-nitro-4-trifluoromethyl-benzene in dimethyl sulfoxide at 20; for 0.341667 h;
Steps:
171
Synthesis 1711 -(2-nitro-4-(trifluoromethyl)phenyl)-1 H-imidazoleA mixture of imidazole (0.997 g, 14.65 mmol) and terf-BuOK (1.722 g, 15.35 mmol) was put under Ar in a 100 mL and dissolved in dry DMSO (15 ml_) to give a colorless solution. After 5 min, 1-fluoro-2-nitro-4-(trifluoromethyl)benzene (2.04 mL, 14.58 mmol) was added within 30 s, immediately leading to a darkening of the rm to black. A temperature rise was also noted. The black solution was stirred at RT for 20 min. Ice water (60 mL) and EtOAc (50 mL) were added, the organic layer was isolated, and the aqueous phase was extracted twice with 20 mL EtOAc. The organic layer was washed with H2O (2 x 30 mL), brine, dried, filtered and evaporated to give the title compound as an orange oil. Yield: 3.66 g (97%).1H-NMR (DMSO-d6), δ (ppm), J (Hz): 7.14 (s, 1 H, Harom), 7.49 (s, 1 H, Harom), 7.97 (d, J=8.4, 1 H1 Harom), 7.99 (s, 1 H, Haro?i), 8.28 (d, J=8.4, 1 H, Harom), 8.59 (s, 1 H, HarOm); 13C- NMR (DMSO-d6), δ (ppm), J (Hz): 120.4, 122.7 (d, JFC=274), 122.9, 129.5 (d, JFC=34), 129.9, 130.0, 130.9, 133.4, 137.4, 144.5; 19F-NMR (DMSO-d6), δ (ppm): -60.8; LC-MS {m/z): 258.1 (M+H, 100), rt=1.37 min.
References:
WO2009/77766,2009,A1 Location in patent:Page/Page column 196