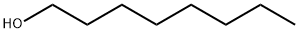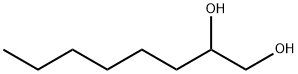
1,2-Octanediol synthesis
- Product Name:1,2-Octanediol
- CAS Number:1117-86-8
- Molecular formula:C8H18O2
- Molecular Weight:146.23

542-69-8
261 suppliers
$9.00/5g

111-66-0
173 suppliers
$10.00/10 mL

1117-86-8
473 suppliers
$8.00/5g
Yield:1117-86-8 42%
Reaction Conditions:
with tert.-butylhydroperoxide;OsO4 in tert-butyl alcohol
Steps:
1 EXAMPLE 1
EXAMPLE 1 Into a 100 ml 3-neck round bottom flask equipped with a magnetic stirrer, reflux condenser, dropping funnel, and thermometer, is charged 0.025 g (0.1 mmole) OsO4, 10.1 g t-butyl alcohol, 3.0 g 1-octene, and 0.037 g (0.2 mmole) n-butyl iodide (co-catalyst I) under continuous agitation and atmospheric pressure. An aqueous solution (2.9 g) containing 70% t-butyl hydroperoxide is then added at atmospheric pressure slowly over a period of 30 minutes at 26° C. to the mixture thereby introducing a total of 22 mmoles of hydroperoxide. The total amount of water in the resulting mixture is 5.9 g. The contents of the flask are warmed to 45° C. as a result of the reaction exotherm during the addition of the hydroperoxide solution. The pH of the resulting solution is about 5. The contents of the reaction flask is then analyzed by gas chromatography upon completion of hydroperoxide addition. The conversion of hydroperoxide is found to be 100%. 1.3 g of 1,2-octanediol is obtained indicating a selectivity to glycol of 42% and a yield of 42%.
References:
Exxon Research & Engineering Co. US4482763, 1984, A

111-66-0
173 suppliers
$10.00/10 mL

1117-86-8
473 suppliers
$8.00/5g
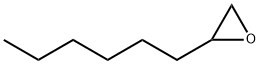
2984-50-1
148 suppliers
$6.00/1g

1117-86-8
473 suppliers
$8.00/5g

111-66-0
173 suppliers
$10.00/10 mL
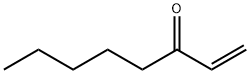
4312-99-6
222 suppliers
$39.00/1g

1117-86-8
473 suppliers
$8.00/5g
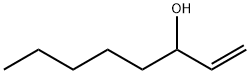
3391-86-4
444 suppliers
$10.00/1g