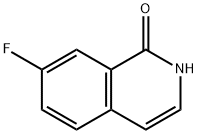
1(2H)-Isoquinolinone,7-fluoro-(9CI) synthesis
- Product Name:1(2H)-Isoquinolinone,7-fluoro-(9CI)
- CAS Number:410086-27-0
- Molecular formula:C9H6FNO
- Molecular Weight:163.15
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

410086-27-0
54 suppliers
$45.00/10mg
Yield: 54%
Reaction Conditions:
with Diphenylmethane at 100 - 280; for 3.5 h;
Steps:
1 7-Fluoroisoquinolin-1(2H)-one (IIc)
A solution of 13.0 g (73.4 mmol, 1.0 eq.) of (E)-3-(4-fluorophenyl)acryloyl azide in 50 mL of diphenylmethane was heated to 100 °C for 30 min. The temperature was subsequently increased to 280 °C and the mixture stirred for a further 3 h. The mixture was allowed to cool to room temperature and the resulting heterogeneous mixture was diluted with 200 mL of n-heptane and stirred for 30 min. The solids were collected by filtration and dried under vacuum. The above detailed reaction was conducted in duplicate and the obtained solid from both batches were combined, triturated with 100 mL of n-heptane, filtered and dried under vacuum to provide 13.0 g (79.8 mmol, 54%) of 7-fluoroisoquinolin-1(2H)-one (IIc). LCMS: m/z found 164.12 [M+H]+, RT = 1.74min; 1H NMR (300 MHz, DMSO-d6): d 11.27 (bs, 1H), 7.74-7.85 (m, 2H), 7.56-7.63 (m, 1H), 7.14-7.21 (m, 1H), 6.59 (d, 1H)
References:
ARBUTUS BIOPHARMA CORPORATION;ARBUTUS BIOPHARMA, INC.;COLE, Andrew G.;FAN, Yi;KULTGEN, Steven;MESAROS, Eugen WO2020/123674, 2020, A1 Location in patent:Page/Page column 286
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

410086-27-0
54 suppliers
$45.00/10mg
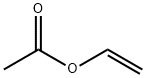
108-05-4
592 suppliers
$4.00/1000g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
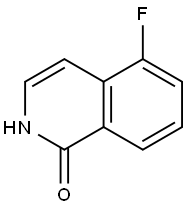
410086-25-8
31 suppliers
inquiry

410086-27-0
54 suppliers
$45.00/10mg
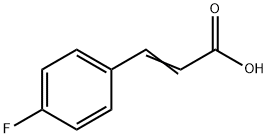
459-32-5
328 suppliers
$6.00/5g

410086-27-0
54 suppliers
$45.00/10mg
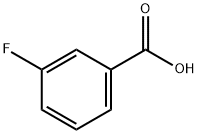
455-38-9
408 suppliers
$11.92/25g

410086-25-8
31 suppliers
inquiry

410086-27-0
54 suppliers
$45.00/10mg