
1(2H)-Naphthalenone, 2-fluoro-3,4-dihydro- synthesis
- Product Name:1(2H)-Naphthalenone, 2-fluoro-3,4-dihydro-
- CAS Number:71019-06-2
- Molecular formula:C10H9FO
- Molecular Weight:164.18
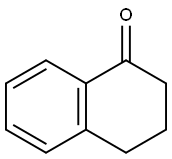
529-34-0
480 suppliers
$6.00/10g

71019-06-2
10 suppliers
inquiry
Yield:71019-06-2 95%
Reaction Conditions:
with 1-(chloromethyl)-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane at 120; for 5 h;Microwave irradiation;
Steps:
4.2.2 General procedure for the synthesis of α-fluoro-α- aryl-α-tetralones
General procedure: In a microwave tube were added a suspension of Selectfluor (0.5314g, 1.5mmol) and α-tetralone (1.0mmol) in PEG400 (2mL) and thermal heated at 120°C. Then, the mixture was allowed to cool to rt, diluted in AcOEt, washed with brine, dried over anhydrous NaSO4, filtered and concentrated under reduced pressure. The crude material was purified by silica gel column with hexane:AcOEt (95:5) as solvent. Compounds 15a [38], 15b [39], 15c [40] are known. Before the first step, in a microwave tube were added a suspension Pd(dba)2 (0.0057 g, 0.01 mmol), tBu3PHBF4 (0.0058 g, 0.02 mmol), NaOH (0.016 g, 0.4 mmol), aryl bromide (0.24 mmol) and compounds 9a-b (0.2 mmol) in a mixture of degassed dioxane/water (4:1, v/v, 4 mL) and heated under argon atmosphere and microwave irradiation (100 W ofinitial power, 100-130 C, 60 min, infrared probe). Then, the mixture was allowed to cool to rt, diluted in AcOEt, washed with saturated NH4Cl solution, dried over anhydrous NaSO4, filtered and concentrated under reduced pressure. The crude material was purified by silica gelcolumn with hexane:AcOEt (95:5) as solvent.
References:
de Souza, Luana G.;Salustiano, Eduardo J.;da Costa, Kelli M.;Costa, Angela T.;Rumjanek, Vivian M.;Domingos, Jorge L.O.;Rennó, Magdalena N.;Costa, Paulo R.R. [Bioorganic Chemistry,2021,vol. 110,art. no. 104790]

62952-26-5
0 suppliers
inquiry

71019-06-2
10 suppliers
inquiry

5736-43-6
0 suppliers
inquiry

71019-06-2
10 suppliers
inquiry
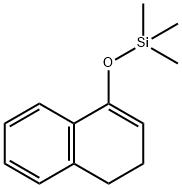
38858-72-9
16 suppliers
$865.28/5G

71019-06-2
10 suppliers
inquiry
![2-Propanamine, N-(2-fluoro-3,4-dihydro-1(2H)-naphthalenylidene)-, [N(E)]- (9CI)](/CAS/20240320/GIF/914801-67-5.gif)
914801-67-5
0 suppliers
inquiry

71019-06-2
10 suppliers
inquiry