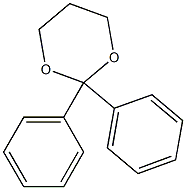
1,3-Dioxane, 2,2-diphenyl- synthesis
- Product Name:1,3-Dioxane, 2,2-diphenyl-
- CAS Number:786-03-8
- Molecular formula:C16H16O2
- Molecular Weight:240.297
Yield: 85%
Reaction Conditions:
with indium(III) triflate;trimethyl orthoformate at 20; for 2 h;
Steps:
4.4. Typical experimental procedure for tandem acetalisation-acetal exchange under solvent-free conditions
General procedure: In(OTf)3 (5.6 mg, 10 μmol, 1 mol %) was added to a mixture of acetophenone (120 mg, 1.00 mmol), trimethyl orthoformate (159 mg, 1.50 mmol) and ethane-1,2-diol (68 mg, 1.10 mmol) and the reaction mixture stirred at room temperature for 1 h. At this time, residual MeOH was removed under reduced pressure and the crude product passed through a short plug of basic alumina, which was washed with hexane (2×2 mL). The solvent was removed under reduced pressure to give the product as a light yellow oil (143 mg, 87%).
References:
Smith, Brendan M.;Kubczyk, Tomasz M.;Graham, Andrew E. [Tetrahedron,2012,vol. 68,# 38,p. 7775 - 7781] Location in patent:experimental part

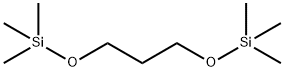
908093-98-1
2 suppliers
inquiry

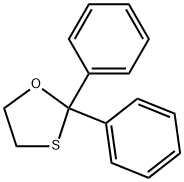
504-63-2
567 suppliers
$9.00/5g
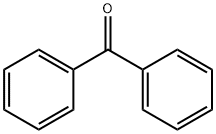
119-61-9
821 suppliers
$5.00/10g

786-03-8
1 suppliers
inquiry