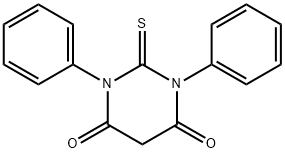
1,3-DIPHENYL-2-THIOBARBITURIC ACID synthesis
- Product Name:1,3-DIPHENYL-2-THIOBARBITURIC ACID
- CAS Number:35221-12-6
- Molecular formula:C16H12N2O2S
- Molecular Weight:296.34
Yield:35221-12-6 95%
Reaction Conditions:
with acetyl chloride at 60; for 0.5 h;
Steps:
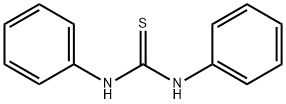
4.1.2 1,3-Diphenylthiobarbituric acid (1a)
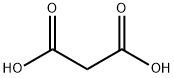
A stirred solution of 59 1,3-diphenylthiourea (1.00mmol; 228mg), 64 malonic acid (1.30mmol; 135mg) and 65 acetyl chloride (3.00mmol; 235mg; 214μL) was heated at 60°C for 30min. The solid 66 product obtained was ground into finer powder, filtered, washed with water and recrystallized from acetic acid; Yield 95%; needle-like yellow crystals; mp 252-253°C (lit [36] 258-259°C); IR υmax (cm-1) 3053, 2895, 1727, 1707, 1594, 1490, 1454, 1381, 1338, 1260, 1212, 1165, 1169, 1037, 1003, 927, 747, 696, 687, 667; 1H NMR (400MHz, CDCl3) δ (ppm) 7.55 (m, 6H, ArCH), 7.21 (d, J= 7.3Hz, 4H, 6′ and 2′-ArCH), 4.10 (s, 2H, 5-CH2); 13C NMR (101MHz, CDCl3) δ (ppm) 181.64 (2-CS), 163.32 (4 and 5-CO), 138.75 (ArC), 129.68 (ArCH), 129.20 (ArCH), 128.64 (ArCH), 41.23 (5-CH2).
References:
Figueiredo, Joana;Serrano, Jo?o L.;Cavalheiro, Eunice;Keurulainen, Leena;Yli-Kauhaluoma, Jari;Moreira, Vania M.;Ferreira, Susana;Domingues, Fernanda C.;Silvestre, Samuel;Almeida, Paulo [European Journal of Medicinal Chemistry,2018,vol. 143,p. 829 - 842]
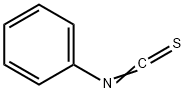
103-72-0
248 suppliers
$10.00/1g

35221-12-6
18 suppliers
inquiry

62-53-3
689 suppliers
$10.00/1g

35221-12-6
18 suppliers
inquiry