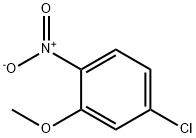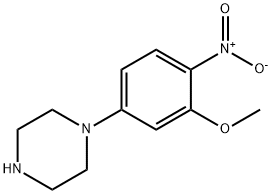
1-(3-methoxy-4-nitrophenyl)piperazine synthesis
- Product Name:1-(3-methoxy-4-nitrophenyl)piperazine
- CAS Number:121278-37-3
- Molecular formula:C11H15N3O3
- Molecular Weight:237.26
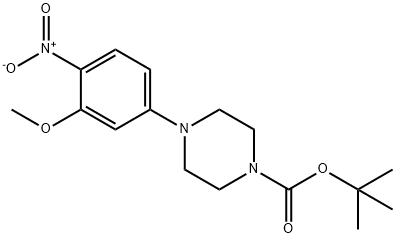
1017782-79-4
41 suppliers
$45.00/10mg

121278-37-3
14 suppliers
inquiry
Yield:121278-37-3 100%
Reaction Conditions:
Stage #1: tert-butyl 4-(3-methoxy-4-nitrophenyl)piperazine-1-carboxylatewith trifluoroacetic acid in dichloromethane at 20;
Stage #2: with sodium hydroxide in dichloromethane;water at 20;
Steps:
B48.B
Step B/lntermediate B50: 1-[3-(methyloxy)-4-nitrophenyl]piperazine; 1-dimethylethyl 4-[3-(methyloxy)-4-nitrophenyl]-1-piperazinecarboxylate (2.25g, 6.67 mmol) was dissolved in methylene chloride (50 ml.) and trifluoroacetic acid (10 ml_). The resulting solution turns immediately dark and was stirred overnight. The solution was concentrated and partitioned between methylene chloride and 2.0N sodium hydroxide. The organic layer was collected and the aqueous layer was saturated by addition of sodium chloride and subsequently backextracted with additional methylene chloride. The combined organic layers were dried over sodium sulfate,
References:
WO2009/20990,2009,A1 Location in patent:Page/Page column 103-104
448-19-1
305 suppliers
$5.00/1g

121278-37-3
14 suppliers
inquiry

110-85-0
588 suppliers
$5.00/5G

121278-37-3
14 suppliers
inquiry