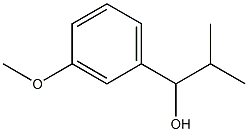
1-(3-Methoxyphenyl)-2-Methylpropan-1-ol synthesis
- Product Name:1-(3-Methoxyphenyl)-2-Methylpropan-1-ol
- CAS Number:61751-33-5
- Molecular formula:C11H16O2
- Molecular Weight:180.24

67-56-1
790 suppliers
$9.00/25ml
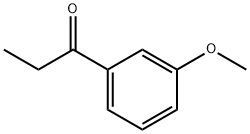
37951-49-8
200 suppliers
$60.00/50mg

61751-33-5
12 suppliers
inquiry
Yield:61751-33-5 86%
Reaction Conditions:
Stage #1: methanol;1-(3-methoxyphenyl)propan-1-onewith [pentamethylcyclopentadienyl*Ir(2,2′-bpyO)(OH)][Na];caesium carbonate at 120; for 12 h;Schlenk technique;
Stage #2: with water at 120; for 12 h;Schlenk technique;
Steps:
4.1. General synthesis of b-methylated secondary alcohols usingmethanol
General procedure: In air, in a 25mL Schlenk tube were added ketone (1 mmol), cat. 9(5.5 mg, 1 mol%), Cs2CO3 (97.8 mg, 0.3 equiv), and MeOH (2 mL). Thereaction mixture was heated at 120 C in an oil bath for 12 h underairtight conditions. After cooling to ambient temperature, 2mL H2Owas added, and the mixture was heated at 120 C for another 12 h.After the given reaction time, the mixture was then cooled to ambienttemperature, concentrated under reduced pressure, and purifiedby flash column chromatography with hexanes/ethyl acetate(50/1-100/1, v/v) to afford the target product, which was identified by NMR analyses. All analytical data of the known compounds areconsistent with those reported in the literature. Some unknowncompounds are detected by NMR and HRMS analyses.
References:
Liu, Shiyuan;Lu, Yao;Song, Ao;Wang, Mingchun;Wang, Rongzhou;Xing, Ling-Bao [Journal of Catalysis,2022,vol. 407,p. 90 - 96]
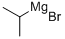
920-39-8
230 suppliers
$85.70/4x25ml
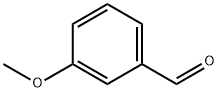
591-31-1
428 suppliers
$10.00/5g

61751-33-5
12 suppliers
inquiry