
Methanol synthesis
- Product Name:Methanol
- CAS Number:67-56-1
- Molecular formula:CH4O
- Molecular Weight:32.04

56-81-5
1768 suppliers
$5.00/25g

67-56-1
787 suppliers
$9.00/25ml
Yield:67-56-1 100%
Reaction Conditions:
with hydrogen;10%Ru/C in water at 100; under 15001.5 Torr; for 2 h;Product distribution / selectivity;Autoclave;
Steps:
2
Example 2 - Study of the effect of supports and base promoterThe reaction of Example 1 was repeated using various different materials in place of the 10% Ru / graphite catalyst, to study the effect of supports and base promoter. The results are shown in Table 2.Table 2 Reaction conditions: 25 mg of catalyst, 5 vol. % glycerol solution, 20 bar hydrogen, 100 0C, 2 hours.
References:
WO2009/130452,2009,A1 Location in patent:Page/Page column 18
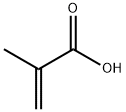
79-41-4
538 suppliers
$14.00/5g
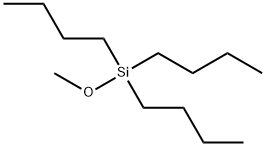
15811-64-0
11 suppliers
inquiry

67-56-1
787 suppliers
$9.00/25ml
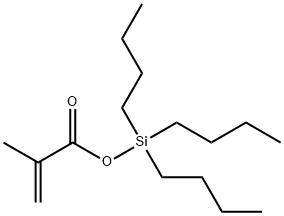
22414-62-6
21 suppliers
inquiry
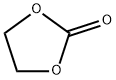
96-49-1
535 suppliers
$6.00/100g

67-56-1
787 suppliers
$9.00/25ml
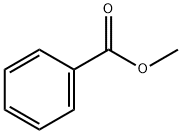
93-58-3
508 suppliers
$14.00/25mL

67-56-1
787 suppliers
$9.00/25ml

100-51-6
1412 suppliers
$5.00/100g
