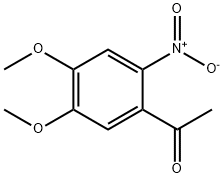
1-(4,5-DIMETHOXY-2-NITRO-PHENYL)-ETHANONE synthesis
- Product Name:1-(4,5-DIMETHOXY-2-NITRO-PHENYL)-ETHANONE
- CAS Number:4101-32-0
- Molecular formula:C10H11NO5
- Molecular Weight:225.2
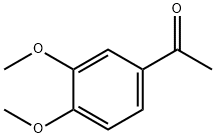
1131-62-0

4101-32-0
General procedure for the synthesis of 1-(4,5-dimethoxy-2-nitrophenyl)ethanone from 3,4-dimethoxyacetophenone: (1) Nitration step: a solution was prepared by dissolving 3,4-dimethoxyacetophenone (1,500 g) in 17% nitric acid (1,400 g) at 5 to 10 °C, followed by slow dropwise addition to a solution consisting of 67% nitric acid (8430 g) and sodium nitrite (18 g) (18 g). The titration process was controlled to be completed within 2 to 3 hours. After the dropwise addition, the reaction solution was continued to be stirred at 5 to 10°C for 1 to 2 hours. Cold water (7.5 L) was added to dilute the reaction mixture and stirred for 30 minutes. The reaction mixture was filtered and the filter cake was washed with water (30 L). The solid product obtained by filtration was re-suspended in water (7.5 L), neutralized with aqueous sodium bicarbonate to pH neutral, filtered again and washed with water (7 L). Finally, the product was dried under reduced pressure to afford 3,4-dimethoxy-6-nitroacetophenone (2164 g, 87.9% yield). The structure of the product was confirmed by 1H-NMR (400 MHz, CDCl3): δ 2.50 (s, 3H), 3.97 (s, 3H), 3.99 (s, 3H), 6.76 (s, 1H), 7.62 (s, 1H).

1131-62-0
328 suppliers
$14.00/5g

4101-32-0
112 suppliers
$22.39/1G
Yield: 89.4%
Reaction Conditions:
with nitric acid in dichloromethane at -10; for 2 h;
Steps:
1.2. General procedure for preparation of(1)
A mixture of 3,4-dimethoxyacetophenone (100.0 g, 0.6 mol) in CH2Cl2(500 mL, 5 v/w) was stirred at -10oC.98% HNO3(70 mL) was added slowly dropwise and then was stirred for 2 h. The reaction solution was poured into ice water with vigorous stirring, and the aqueous phase was extracted with dichloromethane, washed with water, dried over Na2SO4and then concentrated under reduced pressure to give compound1as a yellow solid in 89.4% yield.
References:
Xu, Qiaoling;Dai, Baozhu;Li, Zhiwei;Xu, Le;Yang, Di;Gong, Ping;Hou, Yunlei;Liu, Yajing [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 19,art. no. 126630] Location in patent:supporting information
498-02-2
484 suppliers
$5.00/1g

4101-32-0
112 suppliers
$22.39/1G
91-16-7
596 suppliers
$13.00/25g

4101-32-0
112 suppliers
$22.39/1G
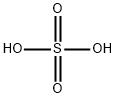
7664-93-9
0 suppliers
$21.64/1003
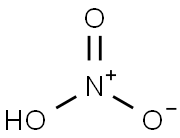
7697-37-2
0 suppliers
$13.00/25g

1131-62-0
328 suppliers
$14.00/5g

4101-32-0
112 suppliers
$22.39/1G

7697-37-2
0 suppliers
$13.00/25g

1131-62-0
328 suppliers
$14.00/5g

4101-32-0
112 suppliers
$22.39/1G