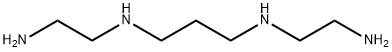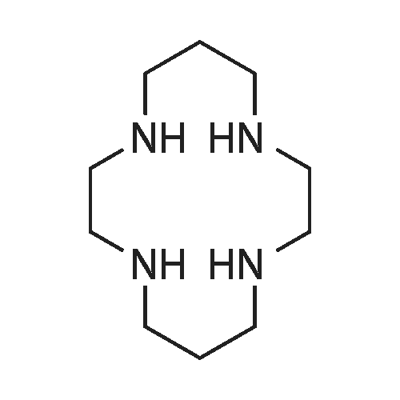
1,4,8,11-TETRAAZACYCLOTETRADECANE synthesis
- Product Name:1,4,8,11-TETRAAZACYCLOTETRADECANE
- CAS Number:295-37-4
- Molecular formula:C10H24N4
- Molecular Weight:200.32
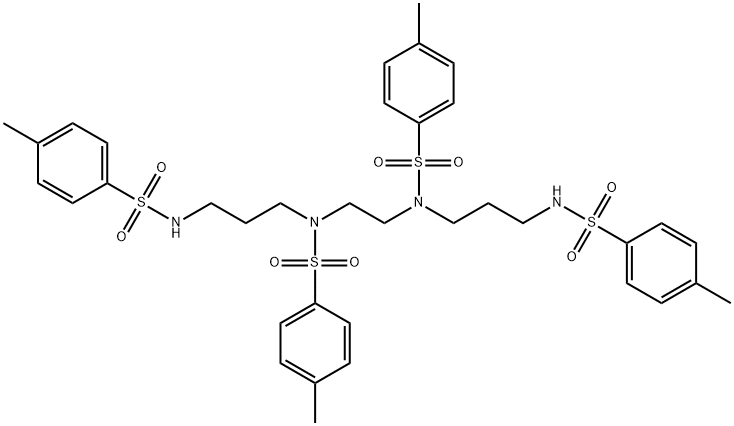
![1,4,8,11-Tetrakis[(4-methylphenyl)sulfonyl]-1,4,8,11-tetraazacyclotetradecane](/CAS2/GIF/71089-74-2.gif)
71089-74-2

295-37-4
The general procedure for the synthesis of 1,4,8,11-tetrakis[(4-methylphenyl)sulfonyl]-1,4,8,11-tetraazacyclotetradecane from 1,4,8,11-tetrakis(4-methylphenyl)sulfonyl]-1,4,8,11-tetraazacyclotetradecane is as follows: 1,4,8,11-tetra-tosyl-1,4,8,11-tetraazacyclotetradecane (13 mmol) was dissolved in 90% concentrated sulfuric acid, and the reaction was carried out at 100 °C The reaction was stirred for 48 hours at 100 °C. Upon completion of the reaction, the reaction mixture was cooled to 0°C. Subsequently, anhydrous ethanol (120 mL) and anhydrous ether (100 mL) were added dropwise to the reaction mixture to precipitate the solid product. The solid was collected by filtration and the filter cake was washed with a small amount of anhydrous ethanol and anhydrous ether, followed by vacuum drying. The resulting off-white solid was dissolved in 1 mol/L aqueous NaOH (90 mL) and extracted with chloroform (3 x 100 mL). The chloroform layers were combined and dried with anhydrous sodium sulfate overnight. Afterwards, the chloroform was removed by distillation under reduced pressure and the residue was vacuum dried to give a white solid product. Finally, the product was recrystallized from toluene to give 2.2 g of 1,4,8,11-tetraazacyclotetradecane in 85% yield. Mass spectral analysis of the product showed [M]+ = 200.3 m/e, 1H-NMR (400 MHz, CDCl3) δ ppm: 1.72 (t, 4H), 2.23 (s, 4H), 2.68 (s, 8H), 2.75 (t, 8H).
![1,4,8,11-Tetrakis[(4-methylphenyl)sulfonyl]-1,4,8,11-tetraazacyclotetradecane](/CAS2/GIF/71089-74-2.gif)
71089-74-2
44 suppliers
$502.37/5MG

295-37-4
308 suppliers
$18.00/250mg
596820-95-0
0 suppliers
inquiry

295-37-4
308 suppliers
$18.00/250mg
74676-47-4
15 suppliers
inquiry

295-37-4
308 suppliers
$18.00/250mg
![1,4,8,11-Tetraazacyclotetradecane, 1,4,8,11-tetrakis[(trifluoroMethyl)sulfonyl]-](/CAS/GIF/96455-17-3.gif)
96455-17-3
1 suppliers
inquiry

295-37-4
308 suppliers
$18.00/250mg