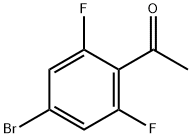
1-(4-BroMo-2,6-difluoro-phenyl)-ethanone synthesis
- Product Name:1-(4-BroMo-2,6-difluoro-phenyl)-ethanone
- CAS Number:746630-34-2
- Molecular formula:C8H5BrF2O
- Molecular Weight:235.03
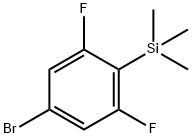
184910-20-1

75-36-5

746630-34-2
1. Anhydrous aluminum trichloride (AlCl3, 16.89 g, 126.7 mmol) was dissolved in dichloromethane (DCM, 130 mL) and acetyl chloride (AcCl, 9 mL, 126.7 mmol) was added slowly at 0 °C. 2. The reaction mixture was stirred at 0 °C for 1 hour. 3. In another vessel, (4-bromo-2,6-difluorophenyl)trimethylsilane (27.48 g, 105.59 mmol) was dissolved in dichloromethane (DCM, 70 mL). 4. The solution from Step 3 was slowly added to the reaction mixture from Step 2 at -80 °C. 5. The reaction mixture was stirred at -80 °C for 16 hours. 6. Upon completion of the reaction, the reaction was quenched with aqueous ammonium chloride at 0 °C and extracted with ether (Et2O). 7. The organic layers were combined and dried with anhydrous magnesium sulfate. 8. The crude product was purified by column chromatography (eluent: ethyl acetate/hexane = 1/10) to afford 1-(4-bromo-2,6-difluorophenyl)ethanone (19.89 g, 74% yield). 9. The structure of the product was investigated by 1H-1H-1 , 1H-2,6-difluorophenylacetonitrile. 9. The structure of the product was confirmed by 1H-NMR (CDCl3, δ): 7.16 (2H, m), 2.58 (3H, s).

184910-20-1
14 suppliers
inquiry

75-36-5
588 suppliers
$17.92/100G

746630-34-2
74 suppliers
$13.00/100mg
Yield: 74%
Reaction Conditions:
Stage #1:acetyl chloride with aluminum (III) chloride in dichloromethane at 0; for 1 h;
Stage #2:(4-bromo-2,6-difluoro-phenyl)-trimethyl-silane in dichloromethane at -80; for 16 h;
Steps:
32.C Step C: 1-(4-bromo-2,6-difluoro-phenyl)ethanone
AlCl3 (16.89 g, 126.7 mmol) was dissolved in DCM (130 mL), and AcCl (9 mL, 126.7 mmol) was added thereto at 0°C. The mixture was stirred for 1 hour. (4-bromo-2,6-difluoro-phenyl)-trimethyl-silane (27.48 g, 105.59 mmol) obtainedin Step B was dissolved in DCM(70 mL) and slowly added thereto at -80°C. The mixture was stirred for 16 hours. Aftertermination of the reaction, the reaction solution was diluted with ammonium chloride aqueous solution at 0°C andextracted with Et2O. The organic layer was dried with anhydrous magnesiumsulfate and purified by column chromatography(eluent, EtOAc/Hex = 1/10) to obtain the title compound (19.89 g, 74 %).NMR: 1H-NMR (CDCl3) δ 7.16(2H, m), 2.58(3H, s)
References:
LG Chem, Ltd.;KIM, Young Kwan;PARK, Sang Yun;JOO, Hyun Woo;CHOI, Eun Sil;PAEK, Seung Yup;KANG, Seung Wan;KIM, Byung Gyu;LEE, Chang Seok;KIM, Sung Wook;LEE, Sang Dae EP3239143, 2017, A2 Location in patent:Paragraph 0140
461-96-1
483 suppliers
$6.00/10g

108-24-7
0 suppliers
$14.00/250ML

746630-34-2
74 suppliers
$13.00/100mg
183065-68-1
270 suppliers
$8.00/5g

746630-34-2
74 suppliers
$13.00/100mg

461-96-1
483 suppliers
$6.00/10g

746630-34-2
74 suppliers
$13.00/100mg