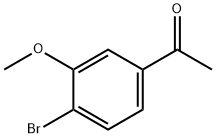
1-(4-BroMo-3-Methoxyphenyl)ethanone synthesis
- Product Name:1-(4-BroMo-3-Methoxyphenyl)ethanone
- CAS Number:50870-44-5
- Molecular formula:C9H9BrO2
- Molecular Weight:229.07
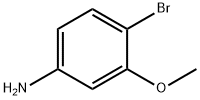
19056-40-7
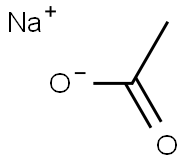
127-09-3

50870-44-5
General procedure for the synthesis of 1-(4-bromo-3-methoxyphenyl)ethanone from 4-bromo-3-methoxyaniline and sodium acetate: commercially available 4-bromo-3-methoxyaniline (10.2 g, 50.6 mmol) was dissolved in a mixture of concentrated hydrochloric acid and water (11 mL of concentrated hydrochloric acid/25 mL of water) that had been pre-cooled to -5 °C, and sodium nitrite (3.48 g) was added in batches. The reaction mixture was stirred at 0 °C for 1 h, followed by slow transfer to an aqueous solution (20.5 mL) containing acetaldoxime (6.02 g), copper sulfate pentahydrate (2.52 g) and sodium acetate trihydrate (36.64 g) at 0 °C. The resulting mixture was stirred between 0°C and 10°C for 2 hours, then 37% hydrochloric acid (23 mL) was added and the mixture was refluxed for 2 hours. After the reaction mixture was cooled to room temperature, it was extracted with heptane and the organic phase was dried with anhydrous magnesium sulfate. The organic solvent was removed by vacuum evaporation to give a residue which was purified by silica gel column chromatography (eluent was dichloromethane/cyclohexane in a gradual ratio from 50/50 to 90/10). Intermediate 681 (5.9 g) in amorphous solid form was finally obtained.1H NMR (400 MHz; CDCl3): δ 7.55 (d, 1H); 7.50 (d, 1H); 7.40 (dd, 1H); 3.95 (s, 3H); 2.60 (s, 3H).IR (cm-1): 1681.

19056-40-7
223 suppliers
$10.00/1g

127-09-3
1177 suppliers
$8.00/1 ea

50870-44-5
53 suppliers
$77.00/250mg
Yield: 5.9 g
Reaction Conditions:
Stage #1:4-bromo-3-methoxyaniline with hydrogenchloride;sodium nitrite in water at -5 - 0; for 1 h;Sandmeyer Reaction;
Stage #2:sodium acetate with Acetaldehyde oxime;copper(II) sulfate in water at 0 - 10; for 2 h;
Stage #3: with hydrogenchloride in water for 2 h;Reflux;
Steps:
To a mixture of commercial 4-bromo-3-methoxyaniline (10.2 g, 50.6 mmoles) in HClcc/H2O (11/25 mL) previously cooled to -5° C. there is added in portions NaNO2 (3.48 g). The reaction mixture is stirred for 1 h at 0° C. before being transferred to a mixture of acetaldoxime (6.02 g), CuSO4 (2.52 g), AcONa.3H2O (36.64 g) in water (20.5 mL) at 0° C. The resulting mixture is stirred between 0° C. and 10° C. for 2 h, and then 37% HCl (23 mL) is added and the mixture is refluxed for 2 hours. After return to ambient temperature, the mixture is extracted with heptane, and the organic phase is dried over MgSO4. Evaporation of the organic phase in vacuo yields a residue, which is chromatographed on silica gel (eluant CH2Cl2/cyclohexane (50/50 to 90/10)). Intermediate 681 (5.9 g) is obtained in the form of an amorphous solid. 1H NMR (400 MHz; CDCl3): δ 7.55 (d, 1H); 7.50 (d, 1H); 7.40 (dd, 1H); 3.95 (s, 3H); 2.60 (s, 3H). IR (cm-1): 1681
References:
LES LABORATOIRES SERVIER;CHIMENTI, Stéfano;COURCHAY, Christine;DESSINGES, Aimee;GELLIBERT, Françoise;GOUMENT, Bertrand;KONNERT, Marc;PEGLION, Jean-Louis;POITEVIN, Christophe;VILAINE, Jean-Paul;VILLENEUVE, Nicole US2017/137385, 2017, A1 Location in patent:Paragraph 0260; 0261; 0262; 0263
56256-15-6
6 suppliers
$420.61/1gm:
917-54-4
184 suppliers
$44.39/50ml

50870-44-5
53 suppliers
$77.00/250mg