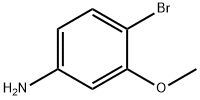
4-BROMO-3-METHOXYANILINE synthesis
- Product Name:4-BROMO-3-METHOXYANILINE
- CAS Number:19056-40-7
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
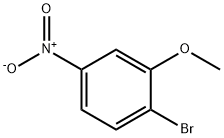
77337-82-7

19056-40-7
General procedure for the synthesis of 4-bromo-3-methoxyaniline from 2-bromo-5-nitroanisole: To a solution of 1-bromo-2-methoxy-4-nitrobenzene (23 g, 99.6 mmol) in tetrahydrofuran (THF, 200 mL) was added ammonium chloride (64 g, 1.2 mol) and zinc powder (78.1 g, 1.2 mol). The reaction mixture was heated to reflux overnight. After completion of the reaction, the mixture was cooled to room temperature, filtered through a bed of diatomite (Celite) and the filtrate was concentrated under reduced pressure. The concentrated residue was partitioned between water (200 mL) and ethyl acetate (200 mL). The organic layer was separated, washed with brine solution (100 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure to give 4-bromo-3-methoxyaniline (17.4 g, 87% yield) as a yellow solid. The product was confirmed by nuclear magnetic resonance hydrogen spectroscopy (1H NMR, DMSO-d6, 400 MHz): δ 7.1 (d, 1H), 6.31 (s, 1H), 6.1 (d, 1H), 5.27 (bs, 2H), 3.72 (s, 3H) ppm.

77337-82-7
179 suppliers
$8.00/1g

19056-40-7
223 suppliers
$10.00/1g
Yield:19056-40-7 89%
Reaction Conditions:
aluminum nickel in methanol;hexane;ethyl acetate
Steps:
1 5-(4-Bromo-3-methoxyphenyl)-N-hydroxy-2-furancarboximidamide Hydrochloride STR4
EXAMPLE 1 5-(4-Bromo-3-methoxyphenyl)-N-hydroxy-2-furancarboximidamide Hydrochloride STR4 2-Bromo-5-nitroanisole (25 g, 0.116 mole) (Fairfield Chemical Co., Blythewood, S.C.) was dissolved in 150 ml of 70:30 methanol/ethyl acetate. Raney nickel (1.0 g) was added to the solution. The mixture was hydrogenated on a Parr shaker for 3 hours, then filtered through Celite. The dark brown filtrate was treated with Darco and concentrated under reduced pressure to a brown solid. The solid was dissolved in 50:50 ethyl acetate/hexane (1 L) and filtered through a 2-inch cake of silica gel (column grade). The resulting solution was concentrated under reduced pressure to a solid yielding 19.3 g (89% yield) of 4-bromo-3-anisidine (O).
References:
Norwich Eaton Pharmaceuticals, Inc. US4882354, 1989, A
536-90-3
298 suppliers
$10.00/5g

19056-40-7
223 suppliers
$10.00/1g

536-90-3
298 suppliers
$10.00/5g
59557-92-5
181 suppliers
$6.00/1g

19056-40-7
223 suppliers
$10.00/1g
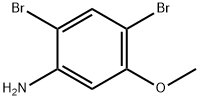
35736-52-8
44 suppliers
$17.00/250mg
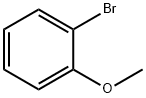
578-57-4
311 suppliers
$6.00/10g
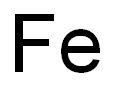
7439-89-6
467 suppliers
$10.00/25g

19056-40-7
223 suppliers
$10.00/1g
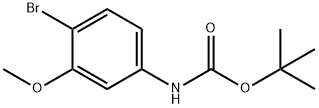
262433-28-3
6 suppliers
inquiry

19056-40-7
223 suppliers
$10.00/1g