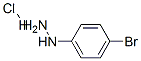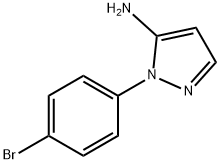
1-(4-Bromophenyl)-1H-pyrazol-5-amine synthesis
- Product Name:1-(4-Bromophenyl)-1H-pyrazol-5-amine
- CAS Number:72194-27-5
- Molecular formula:C9H8BrN3
- Molecular Weight:238.08
Yield: 79 mg
Reaction Conditions:
Stage #1:4-bromophenylhydrazine hydrochloride with sodium hydrogencarbonate in water
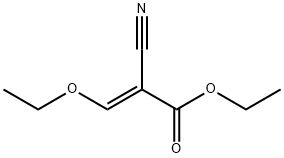
Stage #2:ethyl (ethoxymethylene)cyanoacetate in ethanol for 2 h;Reflux;Further stages;
Steps:
2
The 1-(4-bromophenyl)-1H-pyrazol-5- amine was synthesized starting from 4-(bromophenyl) hydrazine that was obtained from the commercial hydrochloride by treatment with a saturated NaHCO3 aqueous solution (50 mL), followed by dichloromethane extraction (50 mL x3), treatment with anhydrous Na2SO4 and evaporation. To a magnetically stirred solution of 4- (bromophenyl)hydrazine (100 mg, 0.53 mmol, in 5 mL ethanol 5), (E)-ethyl 2-cyano-3-ethoxyacrylate (89.6 mg, (0330) 0.53 mmol) was added and refluxed for 2 h. The reaction mixture was concentrated in vacuo, the residue was suspended in 1:1 methanol/ 2M NaOH aq. solution (5 mL) and refluxed for 1 h. After cooling, the mixture was neutralized with 1M HC1 aq. solution (5 mL) and concentrated in vacuo using a water bath at 40°C. The residue was heated at 180°C for 10 min, suspended in ethanol after cooling and stored overnight at 4°C. The supernatant was recovered and concentrated to give a residue which was stirred in the presence of a NaHCCd solution (10 mL). Extraction with ethyl acetate (10 mL x3), followed by the treatment with anhydrous Na2SO4 of the combined organic phases and concentration in vacuo gave the product (79 mg, 61%), which was used in the following three component reaction. The successful synthesis of 1-(4-bromophenyl)-1H-pyrazol-5-amine was verified by 1H-NMR [δH 7.58(d, J 8.7 Hz, H-3'and H-5'), 7.47(d, J 8.7 Hz, H-2'and H-6'), 7.41(s, H-3), 5.62 (s, (0331) H-4)] and ESIMS (m/z 239.8 [M+H]+).
References:
ISTITUTO NAZIONALE DI FISICA NUCLEARE;UNIVERSITA' DEGLI STUDI DI PERUGIA;UNIVERSITA' DEGLI STUDI DI TRENTO;FONDAZIONE TELETHON WO2021/191883, 2021, A1 Location in patent:Page/Page column 94
1820-80-0
476 suppliers
$5.00/5g
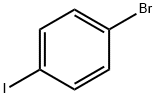
589-87-7
540 suppliers
$10.00/1g

72194-27-5
12 suppliers
$410.00/1g

66000-38-2
2 suppliers
inquiry
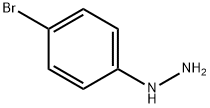
589-21-9
88 suppliers
inquiry

72194-27-5
12 suppliers
$410.00/1g