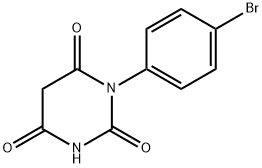
1-(4-bromophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione synthesis
- Product Name:1-(4-bromophenyl)pyrimidine-2,4,6(1H,3H,5H)-trione
- CAS Number:251468-84-5
- Molecular formula:C10H7BrN2O3
- Molecular Weight:283.08
1967-25-5
99 suppliers
$27.30/1g

105-53-3
733 suppliers
$5.00/25g

251468-84-5
6 suppliers
$361.00/100mg
Yield:251468-84-5 89%
Reaction Conditions:
Stage #1: diethyl malonatewith methanol;sodium for 0.0833333 h;
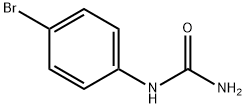
Stage #2: 4-bromophenylurea in methanol; for 6 h;Reflux;
Steps:
1.2 Example 1. Preparation of 3-(4-bromophenyl)-6,6-dihydroxy-5-hydroxyiminohexahydro-2,4-pyrimidinedione (derivative 1)
In the second step, 2 moles (46 g) of 8 metallic sodium are dissolved in 600 ml of anhydrous 9 methanol. 1 mole (160 g) of 10 diethyl malonic ether is added to the obtained solution and stirred for 5 minutes. Then 1 mole (215 g) of N-(4-bromophenyl)urea is added and the mixture is boiled for 6 hours under reflux while stirring. Then the mixture is cooled to 25° C. and 2 L of water is added. The solution is filtered from the precipitate, after which the filtrate is acidified with HCl to pH 1, after which the formed precipitate is filtered and flushed with water. The resulting raw product is treated while stirring with 2 liters of water added to 100 ml of 25% 11 aqueous ammonia, the insoluble residue is discarded, and the solution is acidified with HCl to pH 1, the formed residue is filtered, flushed with water and dried. 250 g of 12 1-(4-bromophenyl)barbituric acid are obtained in the form of a colorless crystalline product with the melting point of 261-263° C. The yield is 89% of the theoretical value.
References:
US2018/338975,2018,A1 Location in patent:Paragraph 0023

106-40-1
458 suppliers
$12.00/5g

251468-84-5
6 suppliers
$361.00/100mg