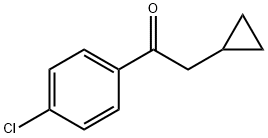
1-(4-chlorophenyl)-2-cyclopropylethanone synthesis
- Product Name:1-(4-chlorophenyl)-2-cyclopropylethanone
- CAS Number:54839-12-2
- Molecular formula:C11H11ClO
- Molecular Weight:194.66
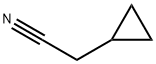
6542-60-5
149 suppliers
$10.00/250mg
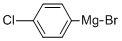
873-77-8
153 suppliers
$61.21/100ml

54839-12-2
21 suppliers
$175.00/50mg
Yield:54839-12-2 21%
Reaction Conditions:
in diethyl ether at 0 - 40; for 1 h;
Steps:
8 [0328]-(4-chlorophenyl)-2-cyclopropylethan-]-one
[0328] ]-(4-chlorophenyl)-2-cyclopropylethan-]-one: To a stirred solution of (4- chlorophenyl) magnesium bromide (1.0 M in ether) (37 mL, 37 mmol) in ether (60 mL) at 0°C under an argon atmosphere was added 2-cyclopropylacetonitrile (3 g, 37 mmol). The reaction mixture was stirred for 1 h at 40 °C. After consumption of the starting material (monitored by TLC), the reaction mixture was diluted with water (100 mL) and extracted with EtOAc (2 x 100 mL). The combined organic extracts were dried over sodium sulfate, filtered and concentrated in vacuo. The crude material was purified by column chromatography using 2% EtOAc: hexane to afford 1 -(4-chlorophenyl)-2-cyclopropylethan-1-one (1.5 g, 21%) as an off-white solid. ‘H NMR (CDC13, 500 MHz): 7.91 (d, 2H), 7.46 (d, 2H), 2.87 (d, 2H), 1.20-1.12 (m, 1H), 0.65-0.60 (m, 2H), 0.23-0.19 (m, 2H); LCMS:95.0%; 194.8 (M+1); (column; Ascentis Express C-18 (50 x 3.0 mm, 2.7 jtm); RT 2.88 mm; mobile phase: 0.025% Aq TFA+5% ACN: ACN+5% 0.025% Aq TFA; T/B%: 0.01/5, 0.5/5, 3/100, 5/100; flow rate: 1.2 mL/min) (Gradient); TLC: 5% EtOAc/ hexane (R1: 0.5).
References:
WO2017/31325,2017,A1 Location in patent:Paragraph 0328