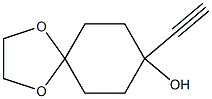
1,4-Dioxaspiro[4.5]decan-8-ol, 8-ethynyl- synthesis
- Product Name:1,4-Dioxaspiro[4.5]decan-8-ol, 8-ethynyl-
- CAS Number:70097-74-4
- Molecular formula:C10H14O3
- Molecular Weight:182.2164
Yield:70097-74-4 100%
Reaction Conditions:
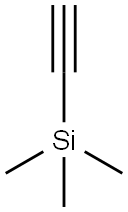
Stage #1: trimethylsilylacetylenewith n-butyllithium in tetrahydrofuran;hexane at -76; for 1 h;
Stage #2: cyclohexanedione monoethylene ketal in tetrahydrofuran;hexane at -74; for 2 h;
Stage #3: with potassium carbonate in methanol at 20; for 2 h;
Steps:
As Intermediate 1, 8-ethinyl-1,4-dioxaspiro[4.5]decan-8-ol: was synthesized by the following procedure.[0335] To a solution of trimethylsilylacetylene (27.1 mL, 0.192 mol) in tetrahydrofuran (300 mL), 2.77 M n-butyllithium (a solution in n-hexane, 69.3 mL, 0.192 mol) was added dropwise at -76Р’В°C for 30 minutes, and the resulting mixture was stirred at the same temperature for 30 minutes. Thereafter, a solution of 1,4-dioxaspiro[4.5]decan-8-one (25.0 g, 0.160 mol) in tetrahydrofuran (100 mL) was added dropwise thereto at -74Р’В°C for 30 minutes, and the resulting mixture was stirred at the same temperature for 1 hour and 30 minutes. The reaction solution was poured into a saturated aqueous ammonium chloride solution, and the resulting solution was extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure.[0336] Methanol (320 mL) was added to the residue to dissolve it, and potassium carbonate (55.3 g, 0.400 mol) was added thereto. The resulting mixture was stirred at room temperature for 2 hours, and the reaction solution was concentrated under reduced pressure. Distilled water was added to the residue, and the resulting solution was extracted with ethyl acetate. The organic layer was washed with distilled water and brine. The organic layer was dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by flash chromatography (silica gel, n-hexane/ethyl acetate) to obtain Intermediate 1 (29.1 g, 0.160 mol, 100%) as a white solid.1H-NMR (400 MHz, CDCl3) Р?Т‘: 1.75-2.03 (9H, m), 2.49 (1H, m), 3.95 (4H, s).ESI-MS: m/z = 165 (M-OH)+
References:
EP2565184,2013,A1 Location in patent:Paragraph 0334-0336
![1,4-Dioxaspiro[4.5]decan-8-ol, 8-[2-(trimethylsilyl)ethynyl]-](/CAS/20210305/GIF/320342-34-5.gif)
320342-34-5
2 suppliers
inquiry
![1,4-Dioxaspiro[4.5]decan-8-ol, 8-ethynyl-](/CAS/20180601/GIF/70097-74-4.gif)
70097-74-4
6 suppliers
inquiry
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
489 suppliers
$5.00/1g
4301-14-8
126 suppliers
$68.50/100ml
![1,4-Dioxaspiro[4.5]decan-8-ol, 8-ethynyl-](/CAS/20180601/GIF/70097-74-4.gif)
70097-74-4
6 suppliers
inquiry
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
489 suppliers
$5.00/1g
![1,4-Dioxaspiro[4.5]decan-8-ol, 8-ethynyl-](/CAS/20180601/GIF/70097-74-4.gif)
70097-74-4
6 suppliers
inquiry