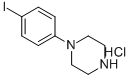
1-(4-IODOPHENYL)PIPERAZINE HYDROCHLORID& synthesis
- Product Name:1-(4-IODOPHENYL)PIPERAZINE HYDROCHLORID&
- CAS Number:624726-35-8
- Molecular formula:C10H13IN2.ClH
- Molecular Weight:324.588

624726-35-8
9 suppliers
$59.70/1g

24424-99-5
817 suppliers
$13.50/25G
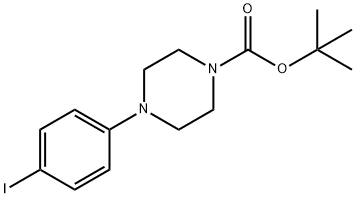
151978-66-4
65 suppliers
$20.00/1g
Yield:151978-66-4 92%
Reaction Conditions:
with sodium hydroxide in water at 20;
Steps:
22
To a solution of 4-iodophenylpiperazine hydrochloride (1.0 g, 3.08 mmol) in water (15 mL) was added sodium hydroxide (246 mg, 6.16 mmol), followed by di- tert-butyl dicarbonate (740 mg, 3.39 mmol). The reaction mixture stirred at room temperature overnight. The next day, the reaction mixture was filtered to collect tert- butyl 4-(4-iodophenyl)piperazine-l-carboxylate as a tan solid (1.1 g, 92% yield), which was washed with water (50 mL) and taken on to the next step without further purification. 1H NMR (600 MHz, CDCl3): δ 7.53 (d, 2H, J =9.0 Hz), 6.68 (d, 2H, J = 9.0 Hz), 3.57 (dd, 4H, J= 5.2, 5.0 Hz), 3.11 (dd, 4H, J= 4.9, 4.9 Hz), 1.49 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 154.9, 151.1, 138.1, 118.8, 82.3, 80.2, 67.3, 49.2, 28.7; HRMS calcd for Ci5H21IN2O5: 389.07205 found 389.07165.
References:
WO2008/83056,2008,A2 Location in patent:Page/Page column 60

624726-35-8
9 suppliers
$59.70/1g
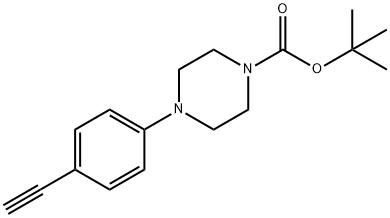
1247003-58-2
0 suppliers
inquiry