
Di-tert-butyl dicarbonate synthesis
- Product Name:Di-tert-butyl dicarbonate
- CAS Number:24424-99-5
- Molecular formula:C10H18O5
- Molecular Weight:218.25
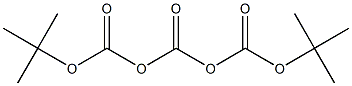
24424-95-1
5 suppliers
inquiry

24424-99-5
871 suppliers
$13.50/25G
Yield:24424-99-5 91 %
Reaction Conditions:
with triethylamine in tetrachloromethane at 25;
Steps:
S3
S51: in the beaker with electromagnetic stirring bar, add 20.0 grams (0.076 moles) of di-tert-butyl tricarbonate dissolved in carbon tetrachloride solution and triethylamine as catalyst, release carbon dioxide immediately and rapidly;S52: stirring at 25° C. for 45 minutes to release carbon dioxide completely, and then adding 35 ml of aqueous solution containing an appropriate amount of citric acid enough to make the water layer weakly acidic;S53: The organic layer was separated, dried with anhydrous magnesium sulfate, concentrated with a rotary evaporator at 25° C., and the residue was distilled under reduced pressure to obtain a colorless liquid of di-tert-butylphenol dicarbonate.
References:
CN115322096,2022,A Location in patent:Paragraph 0063-0067
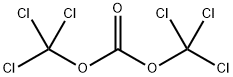
32315-10-9
441 suppliers
$10.00/1g

75-65-0
784 suppliers
$10.00/10ml

24424-99-5
871 suppliers
$13.50/25G
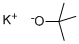
865-47-4
715 suppliers
$14.00/25g

124-63-0
0 suppliers
$10.00/5g

24424-99-5
871 suppliers
$13.50/25G
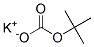
39982-09-7
1 suppliers
inquiry

24424-99-5
871 suppliers
$13.50/25G
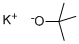
865-47-4
715 suppliers
$14.00/25g
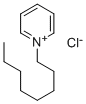
4086-73-1
45 suppliers
inquiry

124-63-0
0 suppliers
$10.00/5g

24424-99-5
871 suppliers
$13.50/25G