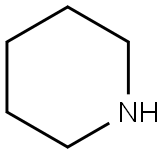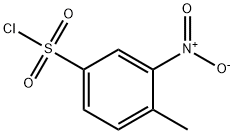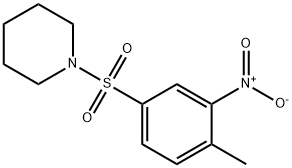
1-[(4-Methyl-3-nitrobenzene)sulfonyl]piperidine synthesis
- Product Name:1-[(4-Methyl-3-nitrobenzene)sulfonyl]piperidine
- CAS Number:91558-67-7
- Molecular formula:C12H16N2O4S
- Molecular Weight:284.33
Yield:91558-67-7 95%
Reaction Conditions:
in dichloromethane at 20;
Steps:
General procedure for the formation of nitrotoluene derivatives 7a-c,e,n-p, 10, 13b-c
General procedure: A solution of commercially available (6, 12) or previously synthesized benzenesulfonylchloride (9) [S1] in CH2Cl2 (8.0 mL for 5 mmol of benzenesulfonylchloride) was added dropwise to the appropriate amine (3 eq) and the reaction was kept under stirring at RT. Once the disappearance of the precursor was verified by TLC (30 min-16 h), the reaction mixture was acidified with 1N aqueous HCl, extracted several times with CH2Cl2 and the combined organic phase was dried over anhydrous sodium sulphate. Evaporation under vacuum of the organic solvent afforded a crude product which was purified by column chromatography over silica gel, to yield the sulfonamidic derivatives.
References:
Granchi, Carlotta;Roy, Sarabindu;Mottinelli, Marco;Nardini, Elisa;Campinoti, Fabio;Tuccinardi, Tiziano;Lanza, Mario;Betti, Laura;Giannaccini, Gino;Lucacchini, Antonio;Martinelli, Adriano;MacChia, Marco;Minutolo, Filippo [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 24,p. 7331 - 7336] Location in patent:supporting information; experimental part
10409-07-1
10 suppliers
inquiry
![1-[(4-Methyl-3-nitrobenzene)sulfonyl]piperidine](/CAS/20150408/GIF/91558-67-7.gif)
91558-67-7
10 suppliers
inquiry