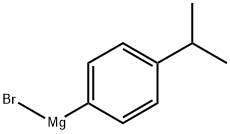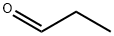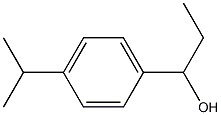
1-(4-propan-2-ylphenyl)propan-1-ol synthesis
- Product Name:1-(4-propan-2-ylphenyl)propan-1-ol
- CAS Number:4397-08-4
- Molecular formula:C12H18O
- Molecular Weight:178.2707
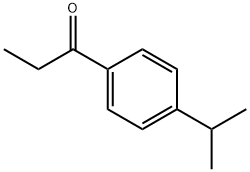
27465-52-7
38 suppliers
$70.00/1g

4397-08-4
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 0 - 20; for 0.5 h;
Steps:
11
Propionyl chloride (11.6 g, 125 mmol) was dropwise added to a suspension of aluminium chloride (16.7 g, 125 mmol) and cumene (18.0 g, 150 mmol) in carbon disulfide (30 mL) at -5° C., and the mixture was stirred for 30 minutes at room temperature. The reaction mixture was poured into water with ice, and the organic layer was separated, washed with an aqueous saturated sodium hydrogencarbonate and water, dried over magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 1-(4-isopropylphenyl)propan-1-one (24.7 g). Sodium borohydride (1.29 g, 34.2 mmol) was added to a solution of the thus-obtained compound (13.0 g, 68.4 mmol) in ethanol (80 mL) with cooling with ice, and the mixture was stirred for 30 minutes at room temperature. Water was added to the reaction mixture, which was then extracted with ethyl acetate. The organic layer was washed with water, dried over magnesium sulfate, filtered, and concentrated under reduced pressure to obtain the title compound (11.5 g, yield 79%). This was oily.1H-NMR (CDCl3) δ: 0.91 (3H, t, J=7.4 Hz), 1.25 (6H, d, J=7.0 Hz), 1.63-1.92 (2H, m), 1.94 (1H, br s), 2.90 (1H, septet, J=7.0 Hz), 4.47-4.61 (1H, m), 7.16-7.29 (4H, m).
References:
US7008950,2006,B1 Location in patent:Page/Page column 40