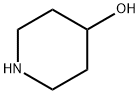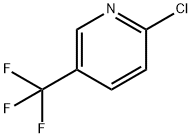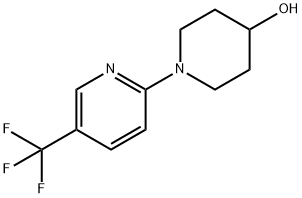
1-[5-(Trifluoromethyl)pyridin-2-yl]piperidin-4-ol synthesis
- Product Name:1-[5-(Trifluoromethyl)pyridin-2-yl]piperidin-4-ol
- CAS Number:832715-03-4
- Molecular formula:C11H13F3N2O
- Molecular Weight:246.23
Yield:832715-03-4 62%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in isopropyl alcohol at 160; for 2.5 h;Irradiation;
Steps:
9.64.1 Example 9. 64 : Preparation of 5'-Bromo-4-[1-(4-methanesulfonyl-phenyl)-1H-pyrazolo [3, 4- D] PYRIMIDIN-4-YLOXY]-3, 4, 5, 6-TETRAHYDRO-2H- [1, 2 ] BIPYRIDINYL (Compound A206) ; and 4- [1- (4-METHANESULFONYL-PHENYL)-LH-PYRAZOLO [3, 4-D] PYRIMIDIN-4-YLOXY]-5 -TRIFLUOROMETHYL-3, 4, 5, 6- tetrahydro-2H-[1,2']bipyridinyl (Compound A208); Step 1 : Preparation of General Procedure for Aromatic Substitution of 2-chloropyridines with 4-hydroxypiperidine
A solution of piperidin-4-ol (100 mg, 0. 99 mmol), desired 2-chlorpyridine (0. 99 mmol, 1. 0 equiv.), and DIPEA (345 PL, 1. 98 mmol) in isopropanol (1. 5 mL) was heated by microwave irradiation at 160 °C for 2. 5 h. The reaction was purified directly by silica gel chromatography to give the desired 3, 4, 5, 6-TETRAHYDRO-2H- [1, 2'] bipyridinyl-4-ol (using 5-bromo-2-chloropyridine, 5'-bromo-3, 4, 5, 6- TETRAHYDRO-2H- [1, 2'] bipyridinyl-4-ol was isolated in 27% yield, 69. 1 mg, 0. 27 MMOL ; using 5- trifluoromethyl-2-chloropyridine, 5'-trifluoromethyl-3, 4, 5, 6-tetrahydro-2H- [1, 2'] bipyridinyl-4-ol was isolated in 62% yield, 150. 1 mg, 0. 61 mmol). HPLC/MS for 5 -BROMO-3, 4, 5, 6-TETRAHYDRO-2H- [1, 2'] bipyridinyl-4-ol : Allteche Prevail C18 column (5μ, 50 X 4. 6 mm), 5% v/v CH3CN (containing 1% v/v TFA) in H20 (containing 1% V/V TFA) gradient to 99% v/v CH3CN in H20, 3. 5 mL/min, tr = 1. 17 min, ESI+ = 258. 9 (M + H). HPLC/MS for 5'-trifluoromethyl-3, 4, 5, 6-tetrahydro-2H- [1, 2'] bipyridinyl-4-ol : ALLTECHNo. PREVAIL C18 column (511, 50 X 4. 6 mm), 5% v/v CH3CN (containing 1% V/V TFA) in H20 (containing 1% v/v TFA) gradient to 99% v/v CH3CN in H20, 3. 5 mL/min, tr = 1. 47 min, ESI+ = 247. 1 (M + H).
References:
WO2005/7658,2005,A2 Location in patent:Page 213