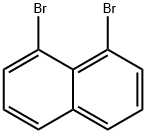
1,8-Dibromonaphthalene synthesis
- Product Name:1,8-Dibromonaphthalene
- CAS Number:17135-74-9
- Molecular formula:C10H6Br2
- Molecular Weight:285.96

![1H-NAPHTHO[1,8-DE][1,2,3]TRIAZINE](/CAS/GIF/204-03-5.gif)
204-03-5
10 suppliers
inquiry

17135-74-9
280 suppliers
$8.00/250mg
Yield:17135-74-9 23%
Reaction Conditions:
Stage #1: 1H-naphtho[1,8-de][1,2,3]triazinewith sulfuric acid;sodium nitrite in water at -5; for 4 h;Inert atmosphere;
Stage #2: with hydrogen bromide;copper(I) bromide in water at -5 - 80; for 3 h;Inert atmosphere;
Steps:
1,8-dibromonaphthalene (1a)
Solid NaNO2 (3.5 g, 0.05 mol) was dissolvedin 20 mL H2O and cooled to 0 oC. The sodium nitrite solution was added drop wise into a solution of 1H-naphtho[1,8-de][1,2,3]triazine (6.8 g, 0.04 mol) in 6.9M H2SO4(100 mL) at -5 C over a period of 2 h with vigorous stirring with a mechanical stirrer.The mixture was stirred at -5 C for an additional 2 h. The solution of CuBr (14.6 g,0.1 mol) in aqueous HBr (47%, 50 mL) was dropwised into the resulting mixture and stirred at -5C for 2 h. The reaction mixture was allowed to warm up to 85C and then stirred for 1 h. The reaction mixture was extracted with dichloromethane and the combined extracts were dried with Na2SO4 and evaporated in vacuum to produce crude product.Flash chromatography on silica gel with petroleum ether gave pure 1a(2.61 g, 23%) as a pale yellow crystal. Melting point: 104-106 C. 1H NMR (300 MHz,CDCl3): 7.92 (d, J = 7.5 Hz, 2H), 7.79 (d, J = 9.0 Hz, 2H), 7.24 (m, 2H).
References:
An, Wenbo;Li, Gaoqiang;Ma, Jun;Tian, Youping;Xu, Feng [Synlett,2014,vol. 25,# 11,art. no. ST-2014-W0213-L,p. 1585 - 1590] Location in patent:supporting information
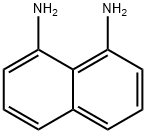
479-27-6
376 suppliers
$6.00/10g

17135-74-9
280 suppliers
$8.00/250mg
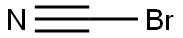
506-68-3
166 suppliers
$10.00/1g
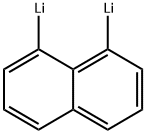
61767-59-7
0 suppliers
inquiry

17135-74-9
280 suppliers
$8.00/250mg

1730-04-7
52 suppliers
$190.00/1g

17135-74-9
280 suppliers
$8.00/250mg
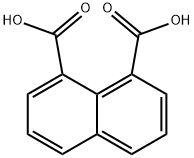
518-05-8
38 suppliers
inquiry

17135-74-9
280 suppliers
$8.00/250mg
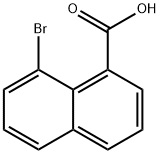
1729-99-3
115 suppliers
$30.00/250mg