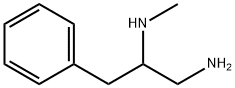
(1-amino-3-phenylpropan-2-yl)(methyl)amine synthesis
- Product Name:(1-amino-3-phenylpropan-2-yl)(methyl)amine
- CAS Number:128899-93-4
- Molecular formula:C10H16N2
- Molecular Weight:164.25
![Carbamic acid, [2-amino-2-oxo-1-(phenylmethyl)ethyl]-, ethyl ester, (S)- (9CI)](/CAS/20210305/GIF/105469-08-7.gif)
105469-08-7
0 suppliers
inquiry
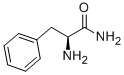
5241-58-7
152 suppliers
$13.00/5g
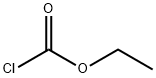
541-41-3
0 suppliers
$21.00/5g

128899-93-4
7 suppliers
inquiry
Yield:-
Reaction Conditions:
with LiAlH4;sodium carbonate in tetrahydrofuran;water;
Steps:
17.25.3 (3)
(3) 1-Amino-(S)-2-N-Methylamino-3-Phenylpropane There was added 10 mmoles (2.36 grams) of N-ethyloxycarbonyl-(L)-phenylalanineamide (produced from (L)-phenylalanineamide and ethyl chloroformate in the presence of Na2 CO3 in an ice bath) in small portions to an ice cooled suspension of 90 mmoles (3.41 grams) of LiAlH4 in 110 ml of tetrahydrofuran. The mixture was heated at reflux for 24 hours. Hydrolysis was carried out under ice ooling with 360 mmoles (6.50 ml) of water. Further working up was analogous to (a). The product was distilled in a high vacuum (10-4 Torr) at 75° C. air bath temperature. Colorless liquid, M.P. of the hydrochloride 160° C.
References:
US4704464,1987,A
![Carbamic acid, [2-amino-2-oxo-1-(phenylmethyl)ethyl]-, ethyl ester, (S)- (9CI)](/CAS/20210305/GIF/105469-08-7.gif)
105469-08-7
0 suppliers
inquiry

128899-93-4
7 suppliers
inquiry